Abstract
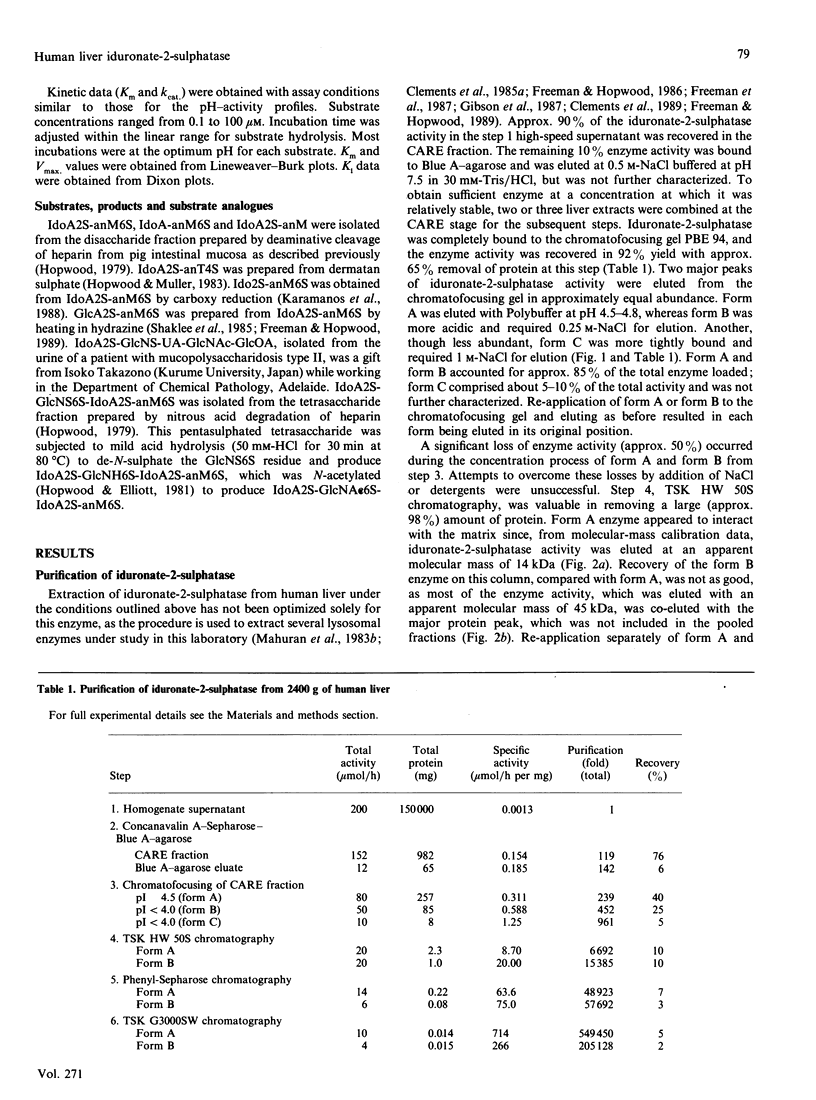
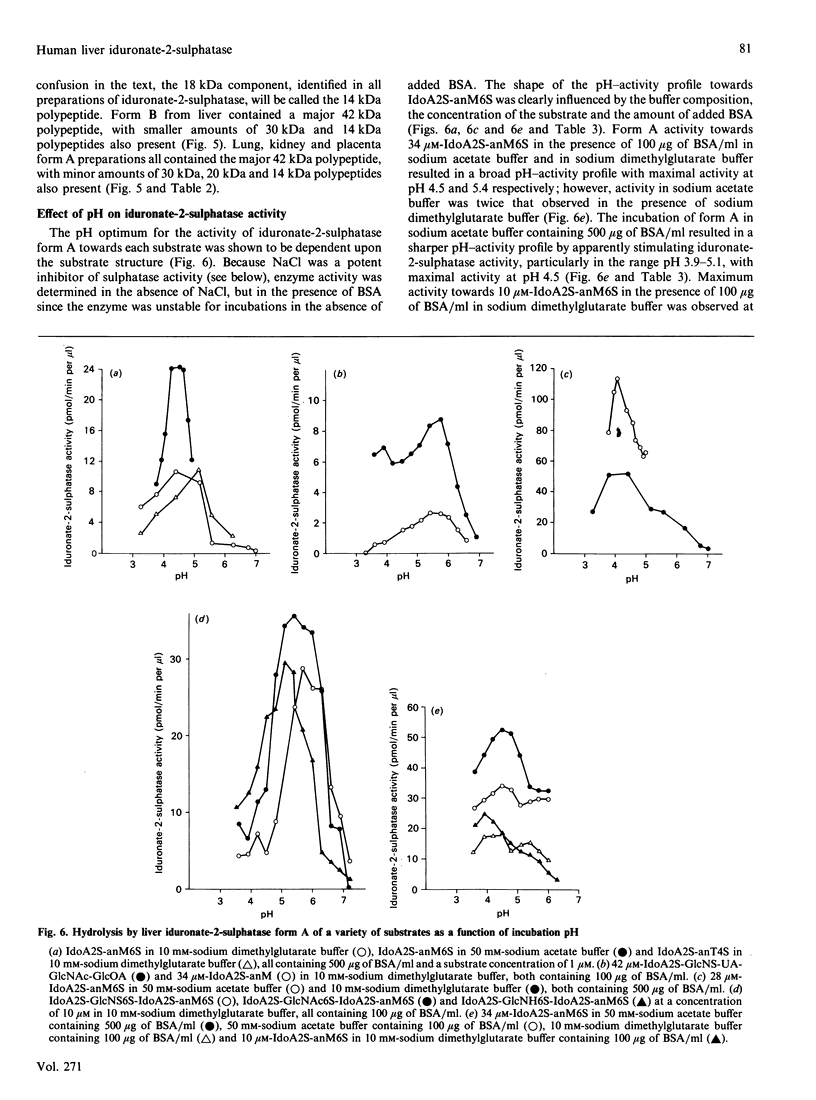
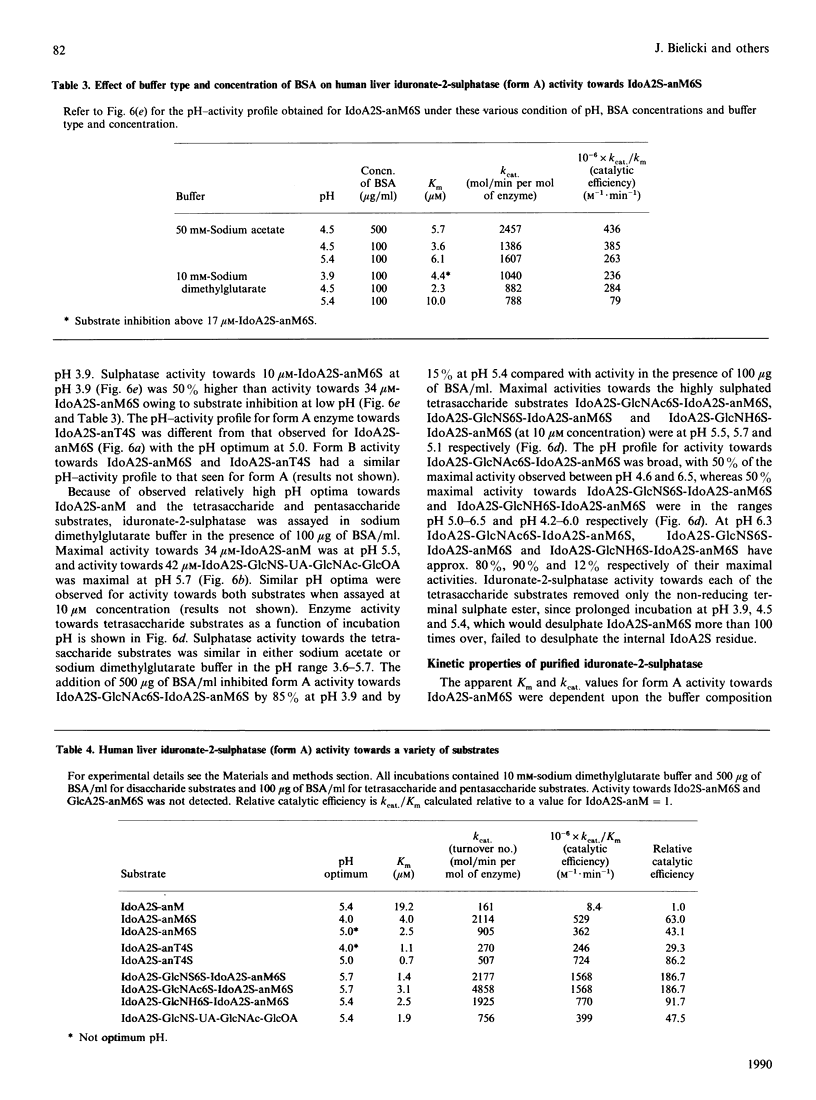
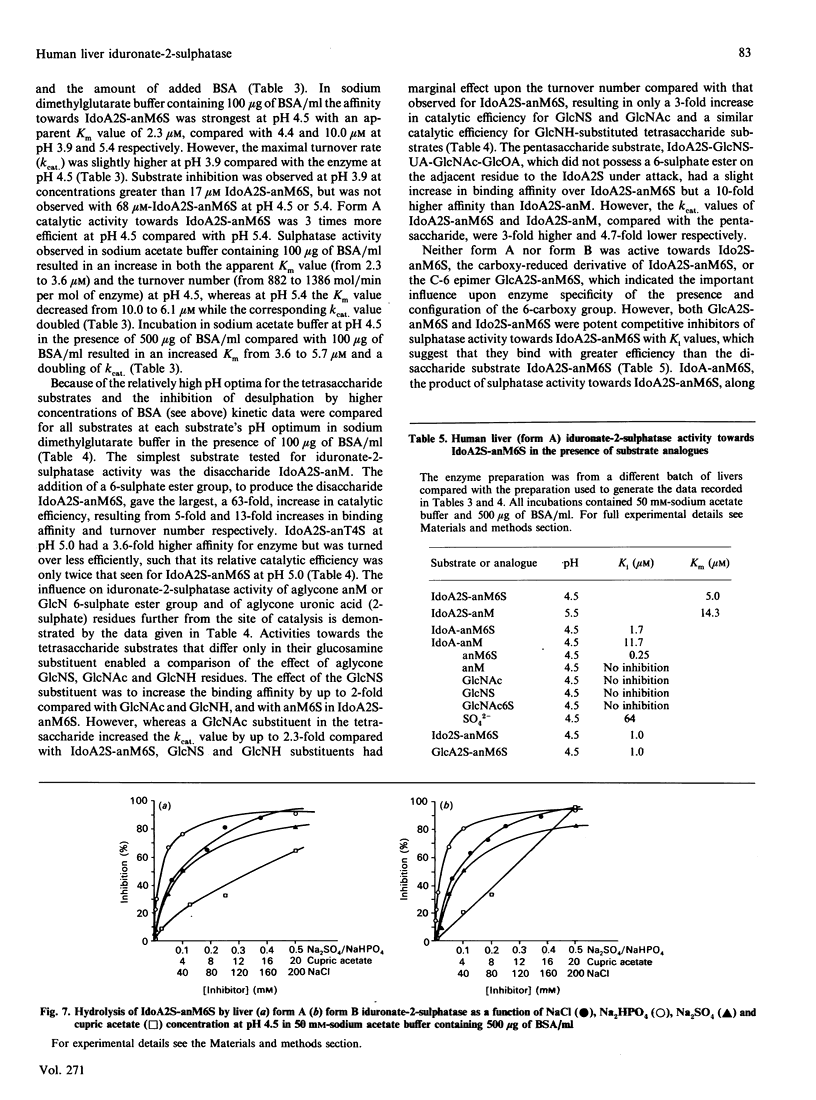
Human iduronate-2-sulphatase (EC 3.1.6.13), which is involved in the lysosomal degradation of the glycosaminoglycans heparan sulphate and dermatan sulphate, was purified more than 500,000-fold in 5% yield from liver with a six-step column procedure, which consisted of a concanavalin A-Sepharose-Blue A-agarose coupled step, chromatofocusing, gel filtration on TSK HW 50S-Fractogel, hydrophobic separation on phenyl-Sepharose CL-4B and size separation on TSK G3000SW Ultrapac. Two major forms were identified. Form A and form B, with pI values of 4.5 and less than 4.0 respectively, separated at the chromatofocusing step in approximately equal amounts of recovered enzyme activity. By gel-filtration methods form A had a native molecular mass in the range 42-65 kDa. When analysed by SDS/PAGE, dithioerythritol-reduced and non-reduced form A and form B consistently contained polypeptides of molecular masses 42 kDa and 14 kDa. Iduronate-2-sulphatase was purified from human kidney, placenta and lung, and form A was shown to have similar native molecular mass and subunit components to those observed for liver enzyme. Both forms of liver iduronate-2-sulphatase were active towards a variety of substrates derived from heparin and dermatan sulphate. Kinetic parameters (Km and Kcat) of form A were determined with a variety of substrates matching structural aspects of the physiological substrates in vivo, namely heparan sulphate, heparin and dermatan sulphate. Substrate with 6-sulphate esters on the aglycone residue adjacent to the iduronic acid 2-sulphate residue being attack were hydrolysed with catalytic efficiencies up to 200 times above that observed for the simplest disaccharide substrate without a 6-sulphated aglycone residue. The effect of incubation pH on enzyme activity towards the variety of substrates evaluated was complex and dependent on substrate aglycone structure, substrate concentration, buffer type and the presence of other proteins. Sulphate and phosphate ions and a number of substrate and product analogues were potent inhibitor of form A and form B enzyme activities.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer I. M., Harper P. S., Wusteman F. S. Multiple forms of iduronate 2-sulphate sulphatase in human tissues and body fluids. Biochim Biophys Acta. 1982 Nov 9;708(2):134–140. doi: 10.1016/0167-4838(82)90213-8. [DOI] [PubMed] [Google Scholar]
- Cantz M., Chrambach A., Bach G., Neufeld E. F. The Hunter corrective factor. Purification and preliminary characterization. J Biol Chem. 1972 Sep 10;247(17):5456–5462. [PubMed] [Google Scholar]
- Clements P. R., Brooks D. A., McCourt P. A., Hopwood J. J. Immunopurification and characterization of human alpha-L-iduronidase with the use of monoclonal antibodies. Biochem J. 1989 Apr 1;259(1):199–208. doi: 10.1042/bj2590199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements P. R., Brooks D. A., Saccone G. T., Hopwood J. J. Human alpha-L-iduronidase. 1. Purification, monoclonal antibody production, native and subunit molecular mass. Eur J Biochem. 1985 Oct 1;152(1):21–28. doi: 10.1111/j.1432-1033.1985.tb09158.x. [DOI] [PubMed] [Google Scholar]
- Clements P. R., Mahuran D. J., Hopwood J. J. Improved concanavalin A-Sepharose elution by specific readsorption of glycoproteins. J Chromatogr. 1983 May 20;261(1):77–82. doi: 10.1016/s0021-9673(01)87920-6. [DOI] [PubMed] [Google Scholar]
- Clements P. R., Muller V., Hopwood J. J. Human alpha-L-iduronidase. 2. Catalytic properties. Eur J Biochem. 1985 Oct 1;152(1):29–34. doi: 10.1111/j.1432-1033.1985.tb09159.x. [DOI] [PubMed] [Google Scholar]
- Daniele A., Di Natale P. Hunter syndrome: presence of material cross-reacting with antibodies against iduronate sulfatase. Hum Genet. 1987 Mar;75(3):234–238. doi: 10.1007/BF00281065. [DOI] [PubMed] [Google Scholar]
- Di Natale P., Daniele A. Iduronate sulfatase from human placenta. Biochim Biophys Acta. 1985 May 8;839(3):258–261. doi: 10.1016/0304-4165(85)90006-6. [DOI] [PubMed] [Google Scholar]
- Di Natale P., Ronsisvalle L. Identification and partial characterization of two enzyme forms of iduronate sulfatase from human placenta. Biochim Biophys Acta. 1981 Sep 15;661(1):106–111. doi: 10.1016/0005-2744(81)90088-7. [DOI] [PubMed] [Google Scholar]
- Freeman C., Clements P. R., Hopwood J. J. Human liver N-acetylglucosamine-6-sulphate sulphatase. Purification and characterization. Biochem J. 1987 Sep 1;246(2):347–354. doi: 10.1042/bj2460347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman C., Hopwood J. J. Human liver N-acetylglucosamine-6-sulphate sulphatase. Catalytic properties. Biochem J. 1987 Sep 1;246(2):355–365. doi: 10.1042/bj2460355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman C., Hopwood J. J. Human liver glucuronate 2-sulphatase. Purification, characterization and catalytic properties. Biochem J. 1989 Apr 1;259(1):209–216. doi: 10.1042/bj2590209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman C., Hopwood J. J. Human liver sulphamate sulphohydrolase. Determinations of native protein and subunit Mr values and influence of substrate agylcone structure on catalytic properties. Biochem J. 1986 Feb 15;234(1):83–92. doi: 10.1042/bj2340083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. J., Saccone G. T., Brooks D. A., Clements P. R., Hopwood J. J. Human N-acetylgalactosamine-4-sulphate sulphatase. Purification, monoclonal antibody production and native and subunit Mr values. Biochem J. 1987 Dec 15;248(3):755–764. doi: 10.1042/bj2480755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Diagnosis of Sanfilippo type A syndrome by estimation of sulfamidase activity using a radiolabelled tetrasaccharide substrate. Clin Chim Acta. 1982 Aug 18;123(3):241–250. doi: 10.1016/0009-8981(82)90168-1. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H., Muller V. J., Saccone G. T. Diagnosis of Maroteaux-Lamy syndrome by the use of radiolabelled oligosaccharides as substrates for the determination of arylsulphatase B activity. Biochem J. 1986 Mar 15;234(3):507–514. doi: 10.1042/bj2340507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Selective depolymerisation of heparin to produce radio-labelled substrates for sulfamidase, 2-acetamido-2-deoxy-alpha-D-glucosidase, acetyl-CoA:2-amino-2-deoxy-alpha-D-glucoside N-acetyltransferase, and 2-acetamido-2-deoxy-D-glucose 6-sulfate sulfatase. Carbohydr Res. 1981 May 1;91(2):165–190. doi: 10.1016/s0008-6215(00)86029-2. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Muller V. J. Selective depolymerisation of dermatan sulfate: production of radiolabelled substrates for alpha-L-iduronidase, sulfoiduronate sulfatase, and beta-D-glucuronidase. Carbohydr Res. 1983 Oct 28;122(2):227–239. doi: 10.1016/0008-6215(83)88334-7. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J. alpha-L-iduronidase, beta-D-glucuronidase, and 2-sulfo-L-iduronate 2-sulfatase: preparation and characterization of radioactive substrates from heparin. Carbohydr Res. 1979 Mar;69:203–216. doi: 10.1016/s0008-6215(00)85765-1. [DOI] [PubMed] [Google Scholar]
- Karamanos N. K., Hjerpe A., Tsegenidis T., Engfeldt B., Antonopoulos C. A. Determination of iduronic acid and glucuronic acid in glycosaminoglycans after stoichiometric reduction and depolymerization using high-performance liquid chromatography and ultraviolet detection. Anal Biochem. 1988 Aug 1;172(2):410–419. doi: 10.1016/0003-2697(88)90463-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lissens W., Zenati A., Liebaers I. Polyclonal antibodies against iduronate 2-sulphate sulphatase from human urine. Biochim Biophys Acta. 1984 Oct 16;801(3):365–371. doi: 10.1016/0304-4165(84)90140-5. [DOI] [PubMed] [Google Scholar]
- Mahuran D., Clements P., Carrella M., Strasberg P. M. A high recovery method for concentrating microgram quantities of protein from large volumes of solution. Anal Biochem. 1983 Mar;129(2):513–516. doi: 10.1016/0003-2697(83)90585-7. [DOI] [PubMed] [Google Scholar]
- Mahuran D., Clements P., Hopwood J. A rapid four column purification of 2-deoxy-D-glucoside-2-sulphamate sulphohydrolase from human liver. Biochim Biophys Acta. 1983 Jun 9;757(3):359–365. doi: 10.1016/0304-4165(83)90062-4. [DOI] [PubMed] [Google Scholar]
- Merle P., Kadenbach B. The subunit composition of mammalian cytochrome c oxidase. Eur J Biochem. 1980 Apr;105(3):499–507. doi: 10.1111/j.1432-1033.1980.tb04525.x. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Shaklee P. N., Glaser J. H., Conrad H. E. A sulfatase specific for glucuronic acid 2-sulfate residues in glycosaminoglycans. J Biol Chem. 1985 Aug 5;260(16):9146–9149. [PubMed] [Google Scholar]
- Tasheva B., Dessev G. Artifacts in sodium dodecyl sulfate-polyacrylamide gel electrophoresis due to 2-mercaptoethanol. Anal Biochem. 1983 Feb 15;129(1):98–102. doi: 10.1016/0003-2697(83)90057-x. [DOI] [PubMed] [Google Scholar]
- Wasteson A., Neufeld E. F. Iduronate sulfatase from human plasma. Methods Enzymol. 1982;83:573–578. doi: 10.1016/0076-6879(82)83053-x. [DOI] [PubMed] [Google Scholar]
- Yutaka T., Fluharty A. L., Stevens R. L., Kihara H. Purification and some properties of human liver iduronate sulfatase. J Biochem. 1982 Feb;91(2):433–441. doi: 10.1093/oxfordjournals.jbchem.a133715. [DOI] [PubMed] [Google Scholar]