Abstract
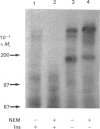
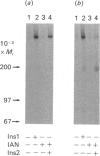
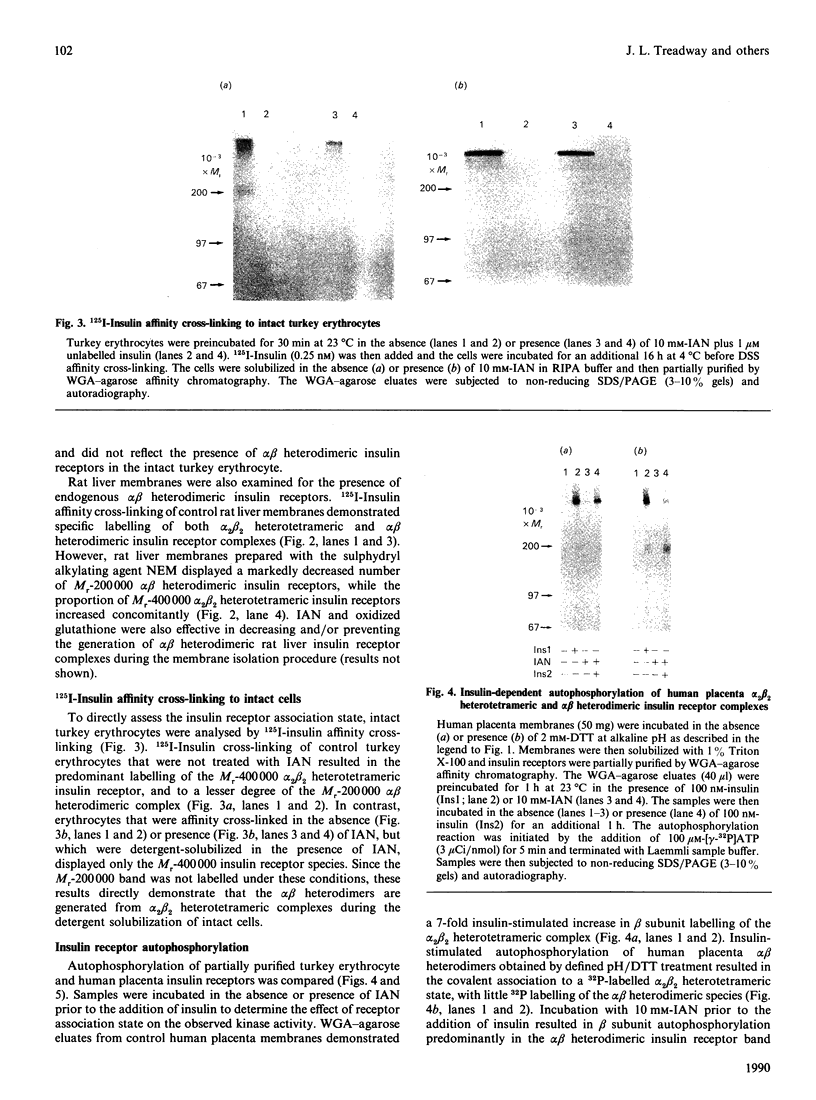
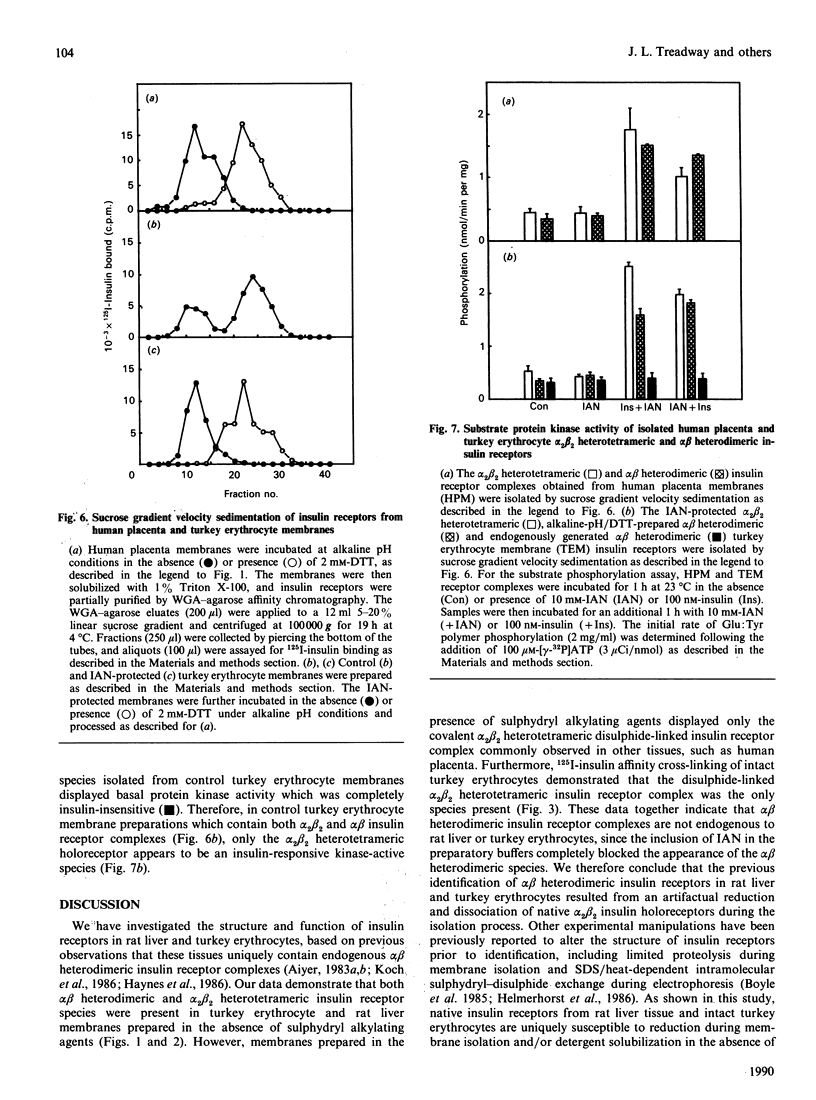
Previous studies have indicated that turkey erythrocyte and rat liver membranes contain endogenous alpha beta heterodimeric insulin receptors in addition to the disulphide-linked alpha 2 beta 2 heterotetrameric complexes characteristic of most cell types. We utilized 125I-insulin affinity cross-linking to examine the structural properties of insulin receptors from rat liver and turkey erythrocyte membranes prepared in the absence and presence of sulphydryl alkylating agents. Rat liver membranes prepared in the absence of sulphydryl alkylating agents displayed specific labelling of Mr 400,000 and 200,000 bands, corresponding to the alpha 2 beta 2 heterotetrameric and alpha beta heterodimeric insulin receptor complexes respectively. In contrast, affinity cross-linking of membranes prepared with iodoacetamide (IAN) or N-ethylmaleimide identified predominantly the alpha 2 beta 2 heterotetrameric insulin receptor complex. Similarly, affinity cross-linking and solubilization of intact turkey erythrocytes in the presence of IAN resulted in exclusive labelling of the alpha 2 beta 2 heterotetrameric insulin receptor complex, whereas in the absence of IAN both alpha 2 beta 2 and alpha beta species were observed. Turkey erythrocyte alpha 2 beta 2 heterotetrameric insulin receptors from IAN-protected membranes displayed a 3-4-fold stimulation of beta subunit autophosphorylation and substrate phosphorylation by insulin, equivalent to that observed in intact human placenta insulin receptors. Turkey erythrocyte alpha beta heterodimeric insulin receptors, prepared by defined pH/dithiothreitol treatment of IAN-protected membranes, were also fully competent in insulin-stimulated protein kinase activity compared with alpha beta heterodimeric human placenta receptors. In contrast, endogenous turkey erythrocyte alpha beta heterodimeric insulin receptors displayed basal protein kinase activity which was insulin-insensitive. These data indicate that native turkey erythrocyte and rat liver insulin receptors are structurally and functionally similar to alpha 2 beta 2 heterotetrameric human placenta insulin receptors. The alpha beta heterodimeric insulin receptors previously identified in these tissues most likely resulted from disulphide bond reduction and denaturation of the alpha 2 beta 2 holoreceptor complexes during membrane preparation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyer R. A. Structural characterization of insulin receptors. I. Hydrodynamic properties of receptors from turkey erythrocytes. J Biol Chem. 1983 Dec 25;258(24):14992–14999. [PubMed] [Google Scholar]
- Aiyer R. A. Structural characterization of insulin receptors. II. Subunit composition of receptors from turkey erythrocytes. J Biol Chem. 1983 Dec 25;258(24):15000–15003. [PubMed] [Google Scholar]
- Boyle T. R., Campana J., Sweet L. J., Pessin J. E. Subunit structure of the purified human placental insulin receptor. Intramolecular subunit dissociation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1985 Jul 15;260(14):8593–8600. [PubMed] [Google Scholar]
- Böni-Schnetzler M., Kaligian A., DelVecchio R., Pilch P. F. Ligand-dependent intersubunit association within the insulin receptor complex activates its intrinsic kinase activity. J Biol Chem. 1988 May 15;263(14):6822–6828. [PubMed] [Google Scholar]
- Böni-Schnetzler M., Rubin J. B., Pilch P. F. Structural requirements for the transmembrane activation of the insulin receptor kinase. J Biol Chem. 1986 Nov 15;261(32):15281–15287. [PubMed] [Google Scholar]
- Böni-Schnetzler M., Scott W., Waugh S. M., DiBella E., Pilch P. F. The insulin receptor. Structural basis for high affinity ligand binding. J Biol Chem. 1987 Jun 15;262(17):8395–8401. [PubMed] [Google Scholar]
- Crettaz M., Jialal I., Kasuga M., Kahn C. R. Insulin receptor regulation and desensitization in rat hepatoma cells. The loss of the oligomeric forms of the receptor correlates with the change in receptor affinity. J Biol Chem. 1984 Sep 25;259(18):11543–11549. [PubMed] [Google Scholar]
- Czech M. P. The nature and regulation of the insulin receptor: structure and function. Annu Rev Physiol. 1985;47:357–381. doi: 10.1146/annurev.ph.47.030185.002041. [DOI] [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y., Choi S., Sakamoto Y., Itakura K. Purification of insulin receptor with full binding activity. J Biol Chem. 1983 Apr 25;258(8):5045–5049. [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y., Kathuria S. The monomeric alpha beta form of the insulin receptor exhibits much higher insulin-dependent tyrosine-specific protein kinase activity than the intact alpha 2 beta 2 form of the receptor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6095–6099. doi: 10.1073/pnas.82.18.6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine I. D. The insulin receptor: molecular biology and transmembrane signaling. Endocr Rev. 1987 Aug;8(3):235–255. doi: 10.1210/edrv-8-3-235. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Itin A. Purification of the insulin receptor from human placenta by chromatography on immobilized wheat germ lectin and receptor antibody. J Biol Chem. 1980 Dec 25;255(24):12066–12072. [PubMed] [Google Scholar]
- Haynes F. J., Helmerhorst E., Yip C. C. The structure of the hepatic insulin receptor and insulin binding. Biochem J. 1986 Oct 1;239(1):127–133. doi: 10.1042/bj2390127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst E., Ng D. S., Moule M. L., Yip C. C. High molecular weight forms of the insulin receptor. Biochemistry. 1986 Apr 22;25(8):2060–2065. doi: 10.1021/bi00356a034. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. The molecular mechanism of insulin action. Annu Rev Med. 1985;36:429–451. doi: 10.1146/annurev.me.36.020185.002241. [DOI] [PubMed] [Google Scholar]
- Koch R., Deger A., Jäck H. M., Klotz K. N., Schenzle D., Krämer H., Kelm S., Müller G., Rapp R., Weber U. Characterization of solubilized insulin receptors from rat liver microsomes. Existence of two receptor species with different binding properties. Eur J Biochem. 1986 Jan 15;154(2):281–287. doi: 10.1111/j.1432-1033.1986.tb09394.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrison B. D., Swanson M. L., Sweet L. J., Pessin J. E. Insulin-dependent covalent reassociation of isolated alpha beta heterodimeric insulin receptors into an alpha 2 beta 2 heterotetrameric disulfide-linked complex. J Biol Chem. 1988 Jun 5;263(16):7806–7813. [PubMed] [Google Scholar]
- O'Hare T., Pilch P. F. Intrinsic kinase activity of the insulin receptor. The intact (alpha 2 beta 2) insulin receptor from rat liver contains a kinase domain with greater intrinsic activity than the intact insulin receptor from human placenta. J Biol Chem. 1989 Jan 5;264(1):602–610. [PubMed] [Google Scholar]
- Stuart C. A. Characterization of a novel insulin receptor from stingray liver. J Biol Chem. 1988 Jun 5;263(16):7881–7886. [PubMed] [Google Scholar]
- Swanson M. L., Pessin J. E. High affinity insulin binding in the human placenta insulin receptor requires alpha beta heterodimeric subunit interactions. J Membr Biol. 1989 Jun;108(3):217–225. doi: 10.1007/BF01871736. [DOI] [PubMed] [Google Scholar]
- Sweet L. J., Morrison B. D., Pessin J. E. Isolation of functional alpha beta heterodimers from the purified human placental alpha 2 beta 2 heterotetrameric insulin receptor complex. A structural basis for insulin binding heterogeneity. J Biol Chem. 1987 May 25;262(15):6939–6942. [PubMed] [Google Scholar]
- Sweet L. J., Morrison B. D., Wilden P. A., Pessin J. E. Insulin-dependent intermolecular subunit communication between isolated alpha beta heterodimeric insulin receptor complexes. J Biol Chem. 1987 Dec 5;262(34):16730–16738. [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]
- Wilden P. A., Morrison B. D., Pessin J. E. Relationship between insulin receptor subunit association and protein kinase activation: insulin-dependent covalent and Mn/MgATP-dependent noncovalent association of alpha beta heterodimeric insulin receptors into an alpha 2 beta 2 heterotetrameric state. Biochemistry. 1989 Jan 24;28(2):785–792. doi: 10.1021/bi00428a056. [DOI] [PubMed] [Google Scholar]
- Wilden P. A., Morrison B. D., Pessin J. E. Wheat germ agglutinin stimulation of alpha beta heterodimeric insulin receptor beta-subunit autophosphorylation by noncovalent association into an alpha 2 beta 2 heterotetrameric state. Endocrinology. 1989 Feb;124(2):971–979. doi: 10.1210/endo-124-2-971. [DOI] [PubMed] [Google Scholar]