Abstract
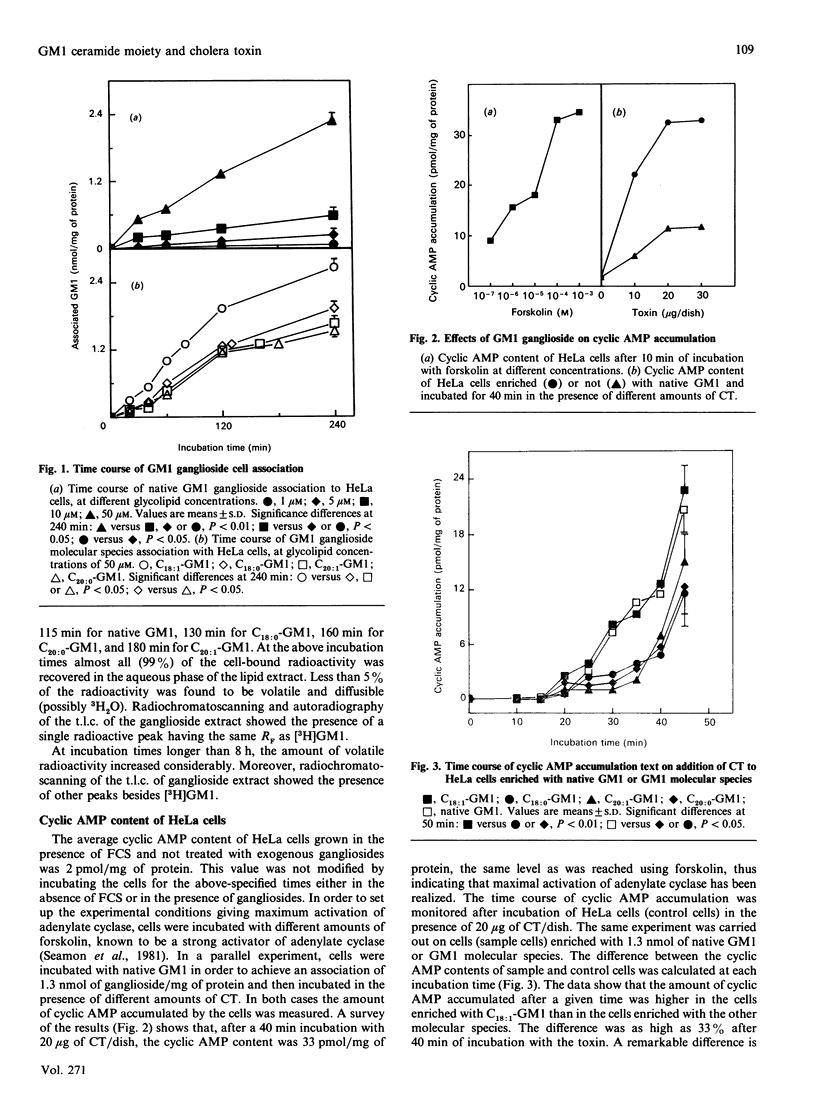
The influence of ceramide composition on the rate of GM1 association to HeLa cells has been investigated by incubating the cells in the presence of either native ganglioside or molecular species carrying highly homogeneous long chain base moieties, fractionated from native GM1. The GM1 ganglioside species carrying the unsaturated C18 long chain base moiety proved to have the fastest rate of association, whereas the saturated species carrying 20 carbon atoms had the slowest rate. After having increased the GM1 cell content (65-fold) by incubation with the various ganglioside species, the cells were incubated with cholera toxin and the time course of cyclic AMP accumulation was monitored. Remarkable differences among cells enriched with the various molecular species were found in the duration of the lag time preceding the accumulation of cyclic AMP, the shortest being displayed by the unsaturated C18 species. Moreover, the amount of cyclic AMP accumulated after a given time of incubation with cholera toxin was significantly higher when the C18:1-GM1 species was present than with native GM1. Fluorescence anisotropy experiments, carried out using the probe 1,3-diphenylhexatriene, show that the GM1 ganglioside ceramide moiety was also modifying the cell membrane fluidity of the host.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chigorno V., Cardace G., Pitto M., Sonnino S., Ghidoni R., Tettamanti G. A radiometric assay for ganglioside sialidase applied to the determination of the enzyme subcellular location in cultured human fibroblasts. Anal Biochem. 1986 Mar;153(2):283–294. doi: 10.1016/0003-2697(86)90094-1. [DOI] [PubMed] [Google Scholar]
- Defrise-Quertain F., Cabiaux V., Vandenbranden M., Wattiez R., Falmagne P., Ruysschaert J. M. pH-dependent bilayer destabilization and fusion of phospholipidic large unilamellar vesicles induced by diphtheria toxin and its fragments A and B. Biochemistry. 1989 Apr 18;28(8):3406–3413. doi: 10.1021/bi00434a040. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Atikkan E. E. Mechanism of action of cholera toxin: effect of receptor density and multivalent binding on activation of adenylate cyclase. J Membr Biol. 1980;54(1):51–60. doi: 10.1007/BF01875376. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Moss J., Osborne J. C., Jr Interaction of choleragen with the oligosaccharide of ganglioside GM1: evidence for multiple oligosaccharide binding sites. Biochemistry. 1978 Feb 21;17(4):711–716. doi: 10.1021/bi00597a024. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Pacuszka T., Hom B., Moss J. Modification of ganglioside GM1. Effect of lipid moiety on choleragen action. J Biol Chem. 1980 Aug 25;255(16):7657–7664. [PubMed] [Google Scholar]
- Fishman P. H. Role of membrane gangliosides in the binding and action of bacterial toxins. J Membr Biol. 1982;69(2):85–97. doi: 10.1007/BF01872268. [DOI] [PubMed] [Google Scholar]
- Ghidoni R., Tettamanti G., Zambotti V. An improved procedure for the in vitro labeling of ganglioside. Ital J Biochem. 1974 Sep-Oct;23(5):320–328. [PubMed] [Google Scholar]
- Ghidoni R., Trinchera M., Venerando B., Fiorilli A., Sonnino S., Tettamanti G. Incorporation and metabolism of exogenous GM1 ganglioside in rat liver. Biochem J. 1986 Jul 1;237(1):147–155. doi: 10.1042/bj2370147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberry R. C., Fishman P. H., Freese E. Morphological changes in cultured mammalian cells: prevention by the calcium ionophore A23187. Cell. 1975 May;5(1):1–9. doi: 10.1016/0092-8674(75)90085-9. [DOI] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fredman P., Svennerholm L. Receptor ganglioside content of three hosts for Sendai virus. MDBK, HeLa, and MDCK cells. Biochim Biophys Acta. 1984 Aug 8;775(1):7–16. doi: 10.1016/0005-2736(84)90228-1. [DOI] [PubMed] [Google Scholar]
- Masserini M., Freire E. Thermotropic characterization of phosphatidylcholine vesicles containing ganglioside GM1 with homogeneous ceramide chain length. Biochemistry. 1986 Mar 11;25(5):1043–1049. doi: 10.1021/bi00353a014. [DOI] [PubMed] [Google Scholar]
- Masserini M., Giuliani A., Palestini P., Acquotti D., Pitto M., Chigorno V., Tettamanti G. Association to HeLa cells and surface behavior of exogenous gangliosides studied with a fluorescent derivative of GM1. Biochemistry. 1990 Jan 23;29(3):697–701. doi: 10.1021/bi00455a015. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Enzymic activity of cholera toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J Biol Chem. 1979 Jul 10;254(13):5855–5861. [PubMed] [Google Scholar]
- Moss J., Fishman P. H., Manganiello V. C., Vaughan M., Brady R. O. Functional incorporation of ganglioside into intact cells: induction of choleragen responsiveness. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1034–1037. doi: 10.1073/pnas.73.4.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Ryan C. A., Lee S. Y., Nadler H. L. Effect of culture conditions on enzyme activities in cultivated human fibroblasts. Exp Cell Res. 1972;71(2):388–392. doi: 10.1016/0014-4827(72)90308-4. [DOI] [PubMed] [Google Scholar]
- Schwarzmann G., Hoffmann-Bleihauer P., Schubert J., Sandhoff K., Marsh D. Incorporation of ganglioside analogues into fibroblast cell membranes. A spin-label study. Biochemistry. 1983 Oct 11;22(21):5041–5048. doi: 10.1021/bi00290a025. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Sonnino S., Chigorno V., Acquotti D., Pitto M., Kirschner G., Tettamanti G. A photoreactive derivative of radiolabeled GM1 ganglioside: preparation and use to establish the involvement of specific proteins in GM1 uptake by human fibroblasts in culture. Biochemistry. 1989 Jan 10;28(1):77–84. doi: 10.1021/bi00427a012. [DOI] [PubMed] [Google Scholar]
- Sonnino S., Ghidoni R., Galli G., Tettamanti G. On the structure of a new, fucose containing ganglioside from pig cerebellum. J Neurochem. 1978 Oct;31(4):947–956. doi: 10.1111/j.1471-4159.1978.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Sonnino S., Ghidoni R., Gazzotti G., Kirschner G., Galli G., Tettamanti G. High performance liquid chromatography preparation of the molecular species of GM1 and GD1a gangliosides with homogeneous long chain base composition. J Lipid Res. 1984 Jun;25(6):620–629. [PubMed] [Google Scholar]
- Stubbs C. D., Smith A. D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984 Jan 27;779(1):89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Suzuki K. Specific radioactive labeling of terminal n-acetylgalactosamine of glycosphingolipids by the galactose oxidase-sodium borohydride method. J Lipid Res. 1972 Sep;13(5):687–690. [PubMed] [Google Scholar]
- Tettamanti G., Bonali F., Marchesini S., Zambotti V. A new procedure for the extraction, purification and fractionation of brain gangliosides. Biochim Biophys Acta. 1973 Jan 19;296(1):160–170. doi: 10.1016/0005-2760(73)90055-6. [DOI] [PubMed] [Google Scholar]
- Tomasi M., Battistini A., Araco A., Roda L. G., D'Agnolo G. The role of the reactive disulfide bond in the interaction of cholera-toxin functional regions. Eur J Biochem. 1979 Feb 1;93(3):621–627. doi: 10.1111/j.1432-1033.1979.tb12862.x. [DOI] [PubMed] [Google Scholar]
- Trinchera M., Wiesmann U., Pitto M., Acquotti D., Ghidoni R. Different metabolic recycling of the lipid components of exogenous sulphatide in human fibroblasts. Biochem J. 1988 Jun 1;252(2):375–379. doi: 10.1042/bj2520375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegandt H., Ziegler W., Staerk J., Kranz T., Ronneberger H. J., Zilg H., Karlsson K. A., Samuelsson B. E. Studies of the ligand binding to cholera toxin, I. The lipophilic moiety of sialoglycolipids. Hoppe Seylers Z Physiol Chem. 1976 Nov;357(11):1637–1646. doi: 10.1515/bchm2.1976.357.2.1637. [DOI] [PubMed] [Google Scholar]
- Yohe H. C., Roark D. E., Rosenberg A. C20-sphingosine as a determining factor in aggregation of gangliosides. J Biol Chem. 1976 Nov 25;251(22):7083–7087. [PubMed] [Google Scholar]


