Abstract
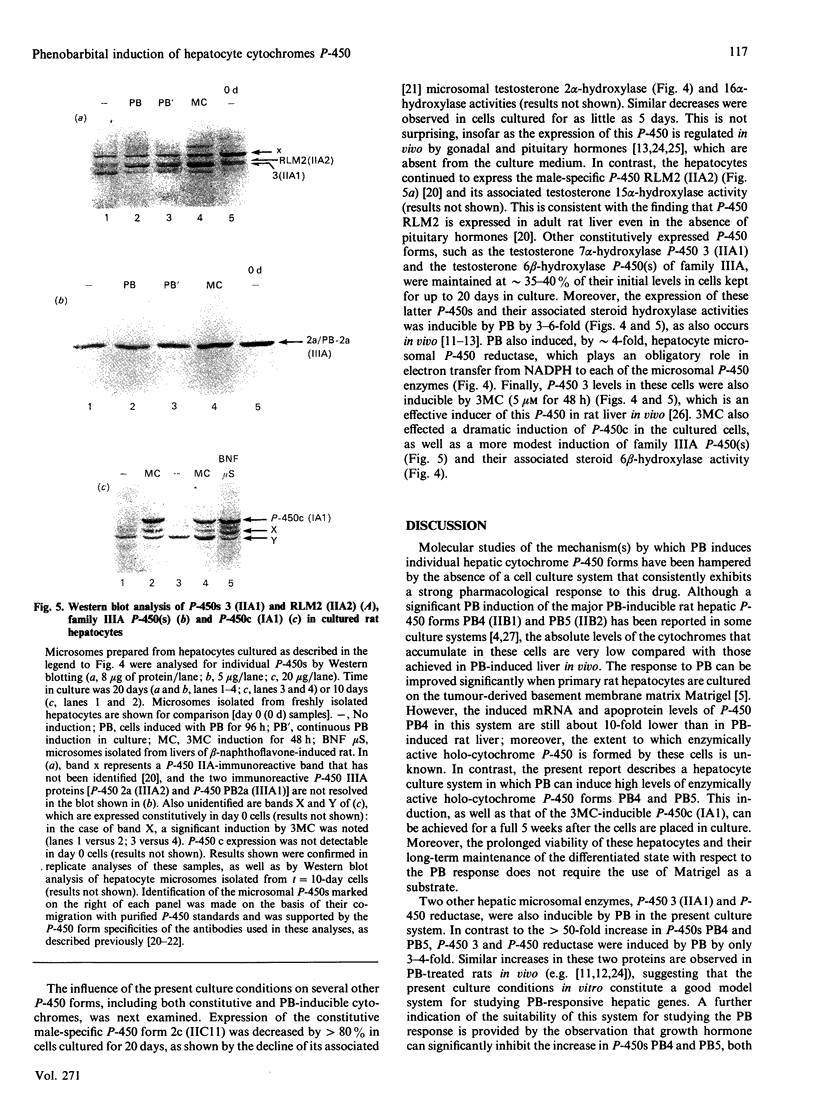
The induction of hepatic cytochromes P-450 by phenobarbital (PB) was studied in rat hepatocytes cultured for up to 5 weeks on Vitrogen-coated plates in serum-free modified Chee's medium then exposed to PB (0.75 mM) for an additional 4 days. Immunoblotting analysis indicated that P-450 forms PB4 (IIB1) and PB5 (IIB2) were induced dramatically (greater than 50-fold increase), up to levels nearly as high as those achieved in PB-induced rat liver in vivo. The newly synthesized cytochrome P-450 was enzymically active, as shown by the major induction of the P-450 PB4-dependent steroid 16 beta-hydroxylase and pentoxyresorufin O-dealkylase activities in the PB-induced hepatocyte microsomes (up to 90-fold increase). PB induction of these P-450s was markedly enhanced by the presence of dexamethasone (50 nM-1 microM), which alone was not an affective inducing agent, and was inhibited by greater than 90% by 10% fetal bovine serum. The PB response was also inhibited (greater than 85%) by growth hormone (250 ng/ml), indicating that this hormone probably acts directly on the hepatocyte when it antagonizes the induction of P-450 PB4 in intact rats. In untreated hepatocytes, P-450 RLM2 (IIA2), P-450 3 (IIA1) and NADPH P-450 reductase levels were substantially maintained in the cultures for 10-20 days. The latter two enzymes were also inducible by PB to an extent (3-4 fold elevation) that is comparable with that observed in the liver in vivo. Moreover, P-450c (IA1) and P-450 3 (IIA1) were highly inducible by 3-methylcholanthrene (5 microM; 48 h exposure) even after 3 weeks in culture. In contrast, the male-specific pituitary-regulated P-450 form 2c (IIC11) was rapidly lost upon culturing the hepatocytes, suggesting that supplementation of appropriate hormonal factors may be necessary for its expression. The present hepatocyte culture system exhibits a responsiveness to drug inducers that is qualitatively and quantitatively comparable with that observed in vivo, and should prove valuable for more detailed investigations of the molecular and mechanistic basis of the response to PB and its modulation by endogenous hormones.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bars R. G., Mitchell A. M., Wolf C. R., Elcombe C. R. Induction of cytochrome P-450 in cultured rat hepatocytes. The heterogeneous localization of specific isoenzymes using immunocytochemistry. Biochem J. 1989 Aug 15;262(1):151–158. doi: 10.1042/bj2620151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Robinson G. S., Bucher N. L., Farmer S. R. Cell-cell and cell-matrix interactions differentially regulate the expression of hepatic and cytoskeletal genes in primary cultures of rat hepatocytes. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2161–2165. doi: 10.1073/pnas.85.7.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D. M., Arenson D. M., Maher J. J., Roll F. J. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987 Mar;79(3):801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee D. O., Boddie A. W., Roth J. A., Holmes E. C., Morton D. L. Production of melanoma-associated antigen(s) by a defined malignant melanoma cell strain grown in chemically defined medium. Cancer Res. 1976 Apr;36(4):1503–1509. [PubMed] [Google Scholar]
- Enat R., Jefferson D. M., Ruiz-Opazo N., Gatmaitan Z., Leinwand L. A., Reid L. M. Hepatocyte proliferation in vitro: its dependence on the use of serum-free hormonally defined medium and substrata of extracellular matrix. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1411–1415. doi: 10.1073/pnas.81.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F. P., Dannan G. A., Wright S. T., Martin M. V., Kaminsky L. S. Purification and characterization of liver microsomal cytochromes p-450: electrophoretic, spectral, catalytic, and immunochemical properties and inducibility of eight isozymes isolated from rats treated with phenobarbital or beta-naphthoflavone. Biochemistry. 1982 Nov 9;21(23):6019–6030. doi: 10.1021/bi00266a045. [DOI] [PubMed] [Google Scholar]
- Guzelian P. S., Li D., Schuetz E. G., Thomas P., Levin W., Mode A., Gustafsson J. A. Sex change in cytochrome P-450 phenotype by growth hormone treatment of adult rat hepatocytes maintained in a culture system on matrigel. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9783–9787. doi: 10.1073/pnas.85.24.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal A. K., Rivkin E., Adesnik M. 5' flanking sequence of the gene for rat hepatic cytochrome P450e. Nucleic Acids Res. 1987 Aug 25;15(16):6755–6755. doi: 10.1093/nar/15.16.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauregui H. O., Hayner N. T., Driscoll J. L., Williams-Holland R., Lipsky M. H., Galletti P. M. Trypan blue dye uptake and lactate dehydrogenase in adult rat hepatocytes--freshly isolated cells, cell suspensions, and primary monolayer cultures. In Vitro. 1981 Dec;17(12):1100–1110. doi: 10.1007/BF02618612. [DOI] [PubMed] [Google Scholar]
- Jauregui H. O., McMillan P. N., Driscoll J., Naik S. Attachment and long term survival of adult rat hepatocytes in primary monolayer cultures: comparison of different substrata and tissue culture media formulations. In Vitro Cell Dev Biol. 1986 Jan;22(1):13–22. doi: 10.1007/BF02623436. [DOI] [PubMed] [Google Scholar]
- Jauregui H. O., McMillan P. N., Hevey K., Naik S. A quantitative analysis of lectin binding to adult rat hepatocyte cell surfaces. In Vitro Cell Dev Biol. 1988 May;24(5):401–412. doi: 10.1007/BF02628491. [DOI] [PubMed] [Google Scholar]
- Kremers P., Letawe-Goujon F., De Graeve J., Duvivier J., Gielen J. E. The expression of different monooxygenases supported by cytochrome P-450 in neonatal rats and in primary fetal hepatocytes in culture. Eur J Biochem. 1983 Dec 15;137(3):603–608. doi: 10.1111/j.1432-1033.1983.tb07868.x. [DOI] [PubMed] [Google Scholar]
- LeBlanc G. A., Waxman D. J. Feminization of rat hepatic P-450 expression by cisplatin. Evidence for perturbations in the hormonal regulation of steroid-metabolizing enzymes. J Biol Chem. 1988 Oct 25;263(30):15732–15739. [PubMed] [Google Scholar]
- Lubet R. A., Mayer R. T., Cameron J. W., Nims R. W., Burke M. D., Wolff T., Guengerich F. P. Dealkylation of pentoxyresorufin: a rapid and sensitive assay for measuring induction of cytochrome(s) P-450 by phenobarbital and other xenobiotics in the rat. Arch Biochem Biophys. 1985 Apr;238(1):43–48. doi: 10.1016/0003-9861(85)90138-9. [DOI] [PubMed] [Google Scholar]
- Macklis J. D., Sidman R. L., Shine H. D. Cross-linked collagen surface for cell culture that is stable, uniform, and optically superior to conventional surfaces. In Vitro Cell Dev Biol. 1985 Mar;21(3 Pt 1):189–194. doi: 10.1007/BF02621357. [DOI] [PubMed] [Google Scholar]
- Mathis J. M., Houser W. H., Bresnick E., Cidlowski J. A., Hines R. N., Prough R. A., Simpson E. R. Glucocorticoid regulation of the rat cytochrome P450c (P450IA1) gene: receptor binding within intron I. Arch Biochem Biophys. 1989 Feb 15;269(1):93–105. doi: 10.1016/0003-9861(89)90090-8. [DOI] [PubMed] [Google Scholar]
- McClellan-Green P. D., Linko P., Yeowell H. N., Goldstein J. A. Hormonal regulation of male-specific rat hepatic cytochrome P-450g (P-450IIC13) by androgens and the pituitary. J Biol Chem. 1989 Nov 15;264(32):18960–18965. [PubMed] [Google Scholar]
- Meehan R. R., Forrester L. M., Stevenson K., Hastie N. D., Buchmann A., Kunz H. W., Wolf C. R. Regulation of phenobarbital-inducible cytochrome P-450s in rat and mouse liver following dexamethasone administration and hypophysectomy. Biochem J. 1988 Sep 15;254(3):789–797. doi: 10.1042/bj2540789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. T., MacGeoch C., Gustafsson J. A. Hormonal and developmental regulation of expression of the hepatic microsomal steroid 16 alpha-hydroxylase cytochrome P-450 apoprotein in the rat. J Biol Chem. 1985 Oct 5;260(22):11895–11898. [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B. The P450 superfamily: updated listing of all genes and recommended nomenclature for the chromosomal loci. DNA. 1989 Jan-Feb;8(1):1–13. doi: 10.1089/dna.1.1989.8.1. [DOI] [PubMed] [Google Scholar]
- Park S. S., Fujino T., West D., Guengerich F. P., Gelboin H. V. Monoclonal antibodies that inhibit enzyme activity of 3-methylcholanthrene-induced cytochrome P-450. Cancer Res. 1982 May;42(5):1798–1808. [PubMed] [Google Scholar]
- Pasco D. S., Boyum K. W., Merchant S. N., Chalberg S. C., Fagan J. B. Transcriptional and post-transcriptional regulation of the genes encoding cytochromes P-450c and P-450d in vivo and in primary hepatocyte cultures. J Biol Chem. 1988 Jun 25;263(18):8671–8676. [PubMed] [Google Scholar]
- Ryan D. E., Thomas P. E., Reik L. M., Levin W. Purification, characterization and regulation of five rat hepatic microsomal cytochrome P-450 isozymes. Xenobiotica. 1982 Nov;12(11):727–744. doi: 10.3109/00498258209038947. [DOI] [PubMed] [Google Scholar]
- Schuetz E. G., Li D., Omiecinski C. J., Muller-Eberhard U., Kleinman H. K., Elswick B., Guzelian P. S. Regulation of gene expression in adult rat hepatocytes cultured on a basement membrane matrix. J Cell Physiol. 1988 Mar;134(3):309–323. doi: 10.1002/jcp.1041340302. [DOI] [PubMed] [Google Scholar]
- Schuetz E. G., Wrighton S. A., Barwick J. L., Guzelian P. S. Induction of cytochrome P-450 by glucocorticoids in rat liver. I. Evidence that glucocorticoids and pregnenolone 16 alpha-carbonitrile regulate de novo synthesis of a common form of cytochrome P-450 in cultures of adult rat hepatocytes and in the liver in vivo. J Biol Chem. 1984 Feb 10;259(3):1999–2006. [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973 Dec;82(2):391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., McQuiddy P., Kasper C. B. Induction of the hepatic mixed-function oxidase system by synthetic glucocorticoids. Transcriptional and post-transcriptional regulation. J Biol Chem. 1987 Jan 5;262(1):326–332. [PubMed] [Google Scholar]
- Steward A. R., Dannan G. A., Guzelian P. S., Guengerich F. P. Changes in the concentration of seven forms of cytochrome P-450 in primary cultures of adult rat hepatocytes. Mol Pharmacol. 1985 Jan;27(1):125–132. [PubMed] [Google Scholar]
- Steward A. R., Wrighton S. A., Pasco D. S., Fagan J. B., Li D., Guzelian P. S. Synthesis and degradation of 3-methylcholanthrene-inducible cytochromes P-450 and their mRNAs in primary monolayer cultures of adult rat hepatocytes. Arch Biochem Biophys. 1985 Sep;241(2):494–508. doi: 10.1016/0003-9861(85)90575-2. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Reik L. M., Ryan D. E., Levin W. Regulation of three forms of cytochrome P-450 and epoxide hydrolase in rat liver microsomes. Effects of age, sex, and induction. J Biol Chem. 1981 Jan 25;256(2):1044–1052. [PubMed] [Google Scholar]
- Turner N. A., Wilson N. M., Jefcoate C. R., Pitot H. C. The expression and metabolic activity of cytochrome P-450 isozymes in control and phenobarbital-induced primary cultures of rat hepatocytes. Arch Biochem Biophys. 1988 May 15;263(1):204–215. doi: 10.1016/0003-9861(88)90629-7. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Dannan G. A., Guengerich F. P. Regulation of rat hepatic cytochrome P-450: age-dependent expression, hormonal imprinting, and xenobiotic inducibility of sex-specific isoenzymes. Biochemistry. 1985 Jul 30;24(16):4409–4417. doi: 10.1021/bi00337a023. [DOI] [PubMed] [Google Scholar]
- Waxman D. J. Interactions of hepatic cytochromes P-450 with steroid hormones. Regioselectivity and stereospecificity of steroid metabolism and hormonal regulation of rat P-450 enzyme expression. Biochem Pharmacol. 1988 Jan 1;37(1):71–84. doi: 10.1016/0006-2952(88)90756-3. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., LeBlanc G. A., Morrissey J. J., Staunton J., Lapenson D. P. Adult male-specific and neonatally programmed rat hepatic P-450 forms RLM2 and 2a are not dependent on pulsatile plasma growth hormone for expression. J Biol Chem. 1988 Aug 15;263(23):11396–11406. [PubMed] [Google Scholar]
- Waxman D. J., Morrissey J. J., LeBlanc G. A. Female-predominant rat hepatic P-450 forms j (IIE1) and 3 (IIA1) are under hormonal regulatory controls distinct from those of the sex-specific P-450 forms. Endocrinology. 1989 Jun;124(6):2954–2966. doi: 10.1210/endo-124-6-2954. [DOI] [PubMed] [Google Scholar]
- Waxman D. J. Rat hepatic cytochrome P-450 isoenzyme 2c. Identification as a male-specific, developmentally induced steroid 16 alpha-hydroxylase and comparison to a female-specific cytochrome P-450 isoenzyme. J Biol Chem. 1984 Dec 25;259(24):15481–15490. [PubMed] [Google Scholar]
- Waxman D. J., Walsh C. Phenobarbital-induced rat liver cytochrome P-450. Purification and characterization of two closely related isozymic forms. J Biol Chem. 1982 Sep 10;257(17):10446–10457. [PubMed] [Google Scholar]
- Yamazoe Y., Shimada M., Murayama N., Kato R. Suppression of levels of phenobarbital-inducible rat liver cytochrome P-450 by pituitary hormone. J Biol Chem. 1987 May 25;262(15):7423–7428. [PubMed] [Google Scholar]
- Zaphiropoulos P. G., Mode A., Ström A., Möller C., Fernandez C., Gustafsson J. A. cDNA cloning, sequence, and regulation of a major female-specific and growth hormone-inducible rat liver cytochrome P-450 active in 15 beta-hydroxylation of steroid sulfates. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4214–4217. doi: 10.1073/pnas.85.12.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]




