Abstract
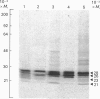
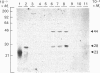
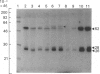
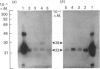
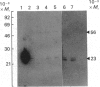
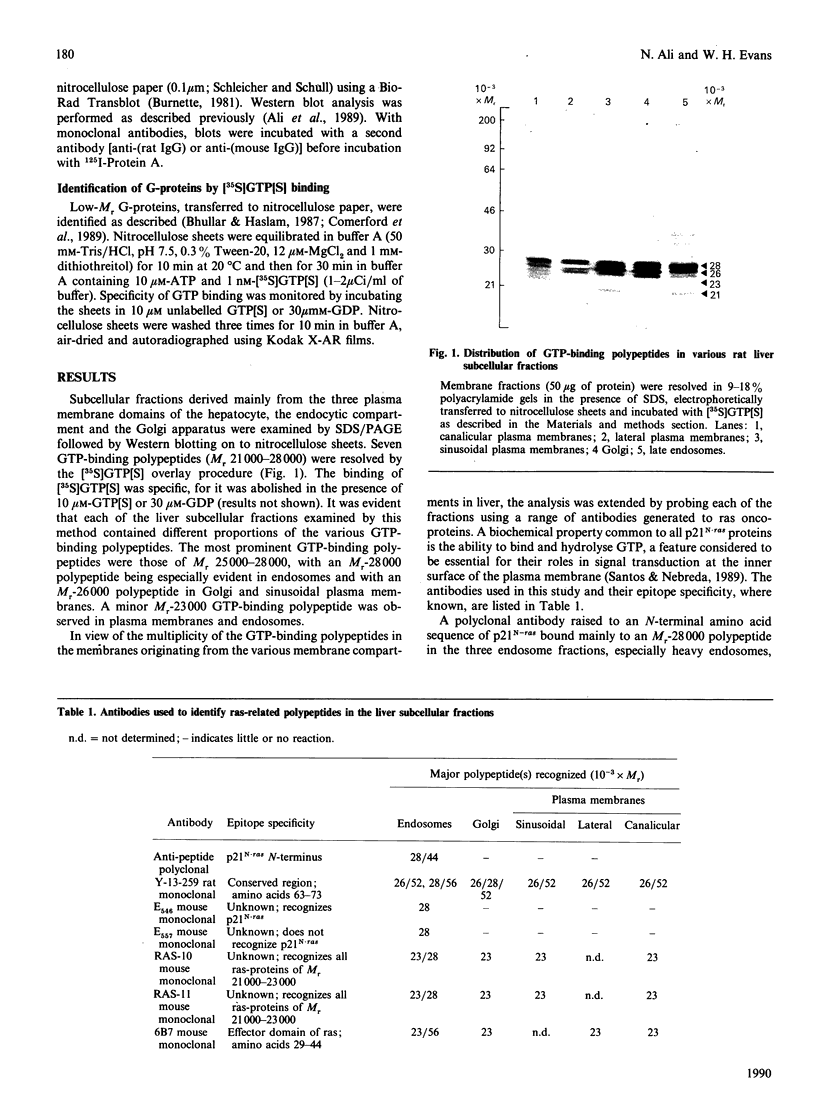
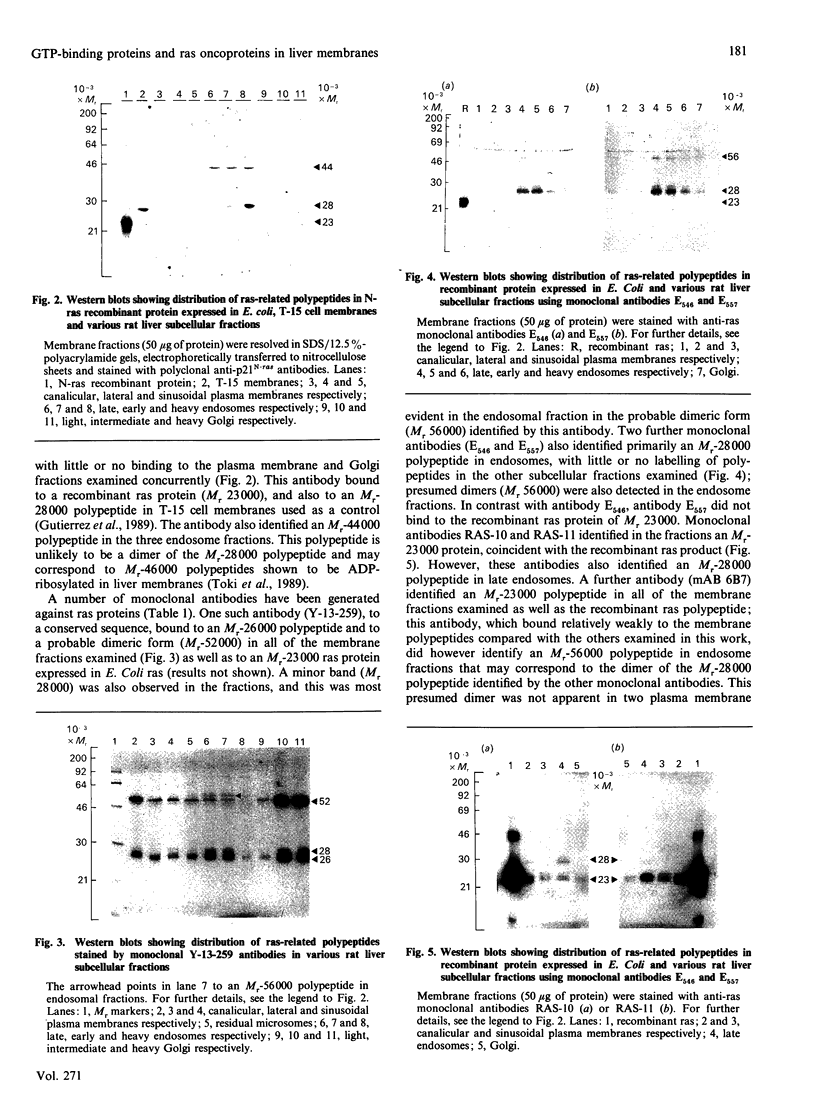
The subcellular distribution in rat liver of polypeptides binding guanosine 5'-[gamma-[35S]thio]triphosphate [( 35S]GTP[S]) and seven antibodies against ras oncoproteins was evaluated. Multiple low-Mr (21,000-28,000) GTP-binding proteins were detected, but their relative distribution among the membrane fractions varied. A more specific compartmentation of polypeptides which bind antibodies generated against ras proteins was evident, with an Mr-28,000 polypeptide and a probable Mr-56,000 dimer, identified by six of the antibodies tested, being confined mainly to endosomes. An Mr-23,000 polypeptide was detected by some of the antibodies in all of the membrane fractions, but especially in the plasma membranes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali N., Milligan G., Evans W. H. Distribution of G-proteins in rat liver plasma-membrane domains and endocytic pathways. Biochem J. 1989 Aug 1;261(3):905–912. doi: 10.1042/bj2610905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Beckers C. J., Balch W. E. Calcium and GTP: essential components in vesicular trafficking between the endoplasmic reticulum and Golgi apparatus. J Cell Biol. 1989 Apr;108(4):1245–1256. doi: 10.1083/jcb.108.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J. Golgi fractions from livers of control and ethanol-intoxicated rats. Enzymic and morphologic properties following rapid isolation. Biochim Biophys Acta. 1979 Aug 23;555(3):493–503. doi: 10.1016/0005-2736(79)90402-4. [DOI] [PubMed] [Google Scholar]
- Bhullar R. P., Haslam R. J. Detection of 23-27 kDa GTP-binding proteins in platelets and other cells. Biochem J. 1987 Jul 15;245(2):617–620. doi: 10.1042/bj2450617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R. Do GTPases direct membrane traffic in secretion? Cell. 1988 Jun 3;53(5):669–671. doi: 10.1016/0092-8674(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Low molecular mass GTP-binding proteins of adrenal chromaffin cells are present on the secretory granule. FEBS Lett. 1989 Mar 13;245(1-2):122–126. doi: 10.1016/0014-5793(89)80204-2. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Solski P. A., Schaeffer J. P., MacDonald M. J., Der C. J. Activation of the cellular proto-oncogene product p21Ras by addition of a myristylation signal. Science. 1989 Mar 24;243(4898):1600–1603. doi: 10.1126/science.2648572. [DOI] [PubMed] [Google Scholar]
- Comerford J. G., Dawson A. P. The effect of limited proteolysis on GTP-dependent Ca2+ efflux and GTP-dependent fusion in rat liver microsomal vesicles. Biochem J. 1989 Mar 15;258(3):823–829. doi: 10.1042/bj2580823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerford J. G., Gibson J. R., Dawson A. P., Gibson I. ras p21 and other Gn proteins are detected in mammalian cell lines by [gamma-35S]GTP gamma S binding. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1269–1274. doi: 10.1016/0006-291x(89)92247-x. [DOI] [PubMed] [Google Scholar]
- Cross S. L. More functions yet: no ras for the weary. The Role of Small GTP-Binding Proteins in Cellular Function, sponsored by the Oncogenes and Mitogens Program Project, University of Virginia Cancer Center, Charlottesville, VA, USA, November 2-3, 1989. New Biol. 1990 Feb;2(2):136–138. [PubMed] [Google Scholar]
- Damonte G., Sdraffa A., Zocchi E., Guida L., Polvani C., Tonetti M., Benatti U., Boquet P., De Flora A. Multiple small molecular weight guanine nucleotide-binding proteins in human erythrocyte membranes. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1398–1405. doi: 10.1016/0006-291x(90)91022-k. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Flint N. Subfractionation of hepatic endosomes in Nycodenz gradients and by free-flow electrophoresis. Separation of ligand-transporting and receptor-enriched membranes. Biochem J. 1985 Nov 15;232(1):25–32. doi: 10.1042/bj2320025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H. Preparation of low-density "endosome" and "endosome"-depleted Golgi fractions from rat liver. Methods Enzymol. 1985;109:246–257. doi: 10.1016/0076-6879(85)09090-5. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988 Jun 3;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Gutierrez L., Magee A. I., Marshall C. J., Hancock J. F. Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. EMBO J. 1989 Apr;8(4):1093–1098. doi: 10.1002/j.1460-2075.1989.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar S., Beatty C., Tsujimoto Y., Croce C. M. The bcl-2 gene encodes a novel G protein. Nature. 1989 Nov 9;342(6246):195–198. doi: 10.1038/342195a0. [DOI] [PubMed] [Google Scholar]
- Kawata M., Kawahara Y., Araki S., Sunako M., Tsuda T., Fukuzaki H., Mizoguchi A., Takai Y. Identification of a major GTP-binding protein in bovine aortic smooth muscle membranes as smg p21, a GTP-binding protein having the same effector domain as ras p21s. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1418–1427. doi: 10.1016/0006-291x(89)91137-6. [DOI] [PubMed] [Google Scholar]
- Kikuchi A., Sasaki T., Araki S., Hata Y., Takai Y. Purification and characterization from bovine brain cytosol of two GTPase-activating proteins specific for smg p21, a GTP-binding protein having the same effector domain as c-ras p21s. J Biol Chem. 1989 Jun 5;264(16):9133–9136. [PubMed] [Google Scholar]
- Klausner R. D. Sorting and traffic in the central vacuolar system. Cell. 1989 Jun 2;57(5):703–706. doi: 10.1016/0092-8674(89)90783-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanoix J., Roy L., Paiement J. Detection of GTP-binding proteins in purified derivatives of rough endoplasmic reticulum. Biochem J. 1989 Sep 1;262(2):497–503. doi: 10.1042/bj2620497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Lacal J. C., Reep B. R., Molina y Vedia L. A ras-related protein is phosphorylated and translocated by agonists that increase cAMP levels in human platelets. Proc Natl Acad Sci U S A. 1989 May;86(9):3131–3134. doi: 10.1073/pnas.86.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga L. S., Diaz R., Colombo M. I., Stahl P. D. GTP gamma S stimulation of endosome fusion suggests a role for a GTP-binding protein in the priming of vesicles before fusion. Cell Regul. 1989 Nov;1(1):113–124. doi: 10.1091/mbc.1.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga L. S., Diaz R., Stahl P. D. Regulatory role for GTP-binding proteins in endocytosis. Science. 1989 Jun 23;244(4911):1475–1477. doi: 10.1126/science.2499930. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A., Kim S., Ueda T., Takai Y. Tissue distribution of smg p25A, a ras p21-like GTP-binding protein, studied by use of a specific monoclonal antibody. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1438–1445. doi: 10.1016/0006-291x(89)90835-8. [DOI] [PubMed] [Google Scholar]
- Nigam S. K. Subcellular distribution of small GTP binding proteins in pancreas: identification of small GTP binding proteins in the rough endoplasmic reticulum. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1296–1299. doi: 10.1073/pnas.87.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos E., Nebreda A. R. Structural and functional properties of ras proteins. FASEB J. 1989 Aug;3(10):2151–2163. doi: 10.1096/fasebj.3.10.2666231. [DOI] [PubMed] [Google Scholar]
- Segev N., Mulholland J., Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988 Mar 25;52(6):915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Shears S. B., Evans W. H., Kirk C. J., Michell R. H. Preferential localization of rat liver D-myo-inositol 1,4,5-trisphosphate/1,3,4,5-tetrakisphosphate 5-phosphatase in bile-canalicular plasma membrane and 'late' endosomal vesicles. Biochem J. 1988 Dec 1;256(2):363–369. doi: 10.1042/bj2560363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal I. S., Gibbs J. B., D'Alonzo J. S., Scolnick E. M. Identification of effector residues and a neutralizing epitope of Ha-ras-encoded p21. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4725–4729. doi: 10.1073/pnas.83.13.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki C., Oda K., Ikehara Y. Demonstration of GTP-binding proteins and ADP-ribosylated proteins in rat liver Golgi fraction. Biochem Biophys Res Commun. 1989 Oct 16;164(1):333–338. doi: 10.1016/0006-291x(89)91722-1. [DOI] [PubMed] [Google Scholar]
- Urumow T., Wieland O. H. A small G-protein involved in phosphatidylinositol-4-phosphate kinase activation. FEBS Lett. 1990 Apr 9;263(1):15–17. doi: 10.1016/0014-5793(90)80694-e. [DOI] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]