Abstract
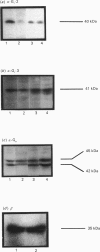
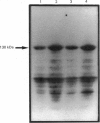
Levels of the G-protein alpha-subunits alpha-Gi-2, alpha-Gi-3 and the 42 kDa, form of alpha-Gs were markedly decreased in hepatocyte membranes from streptozotocin-diabetic animals as compared with normals. In contrast, no detectable changes in alpha-Gi subunits were seen in liver plasma membranes of streptozotocin-diabetic animals, although levels of the 45 kDa form of Gs were increased. G-protein beta subunits in plasma membranes were unaffected by diabetes induction. Analysis of whole-liver RNA indicated that the induction of diabetes had little effect on transcript levels of Gi-3, caused an increase in Gs transcripts and decreased transcript number for Gi-2, albeit to a much lesser extent than was observed upon analysis of hepatocyte RNA. In both hepatocyte and liver plasma membranes, immunoblot analysis showed that levels of the catalytic unit of adenylate cyclase were increased upon induction of diabetes. Under basal conditions, alpha-Gi-2 from hepatocytes of diabetic animals was found to be both phosphorylated to a greater extent than alpha-Gi-2 isolated from hepatocytes of normal animals, and furthermore was resistant to any further phosphorylation upon challenge of hepatocytes with angiotensin, vasopressin or the phorbol ester 12-O-tetradecanoylphorbol 13-acetate. Treatment of isolated plasma membranes from normal, but not diabetic, animals with purified protein kinase C caused the phosphorylation of alpha-Gi-2. Treatment of membranes from diabetic animals with alkaline phosphatase caused the dephosphorylation of alpha-Gi-2 and rendered it susceptible to subsequent phosphorylation with protein kinase C. Low concentrations of the non-hydrolysable GTP analogue guanylyl 5'-imidodiphosphate inhibited adenylate cyclase activity in both hepatocyte and liver plasma membranes from normal, but not diabetic, animals.
Full text
PDF


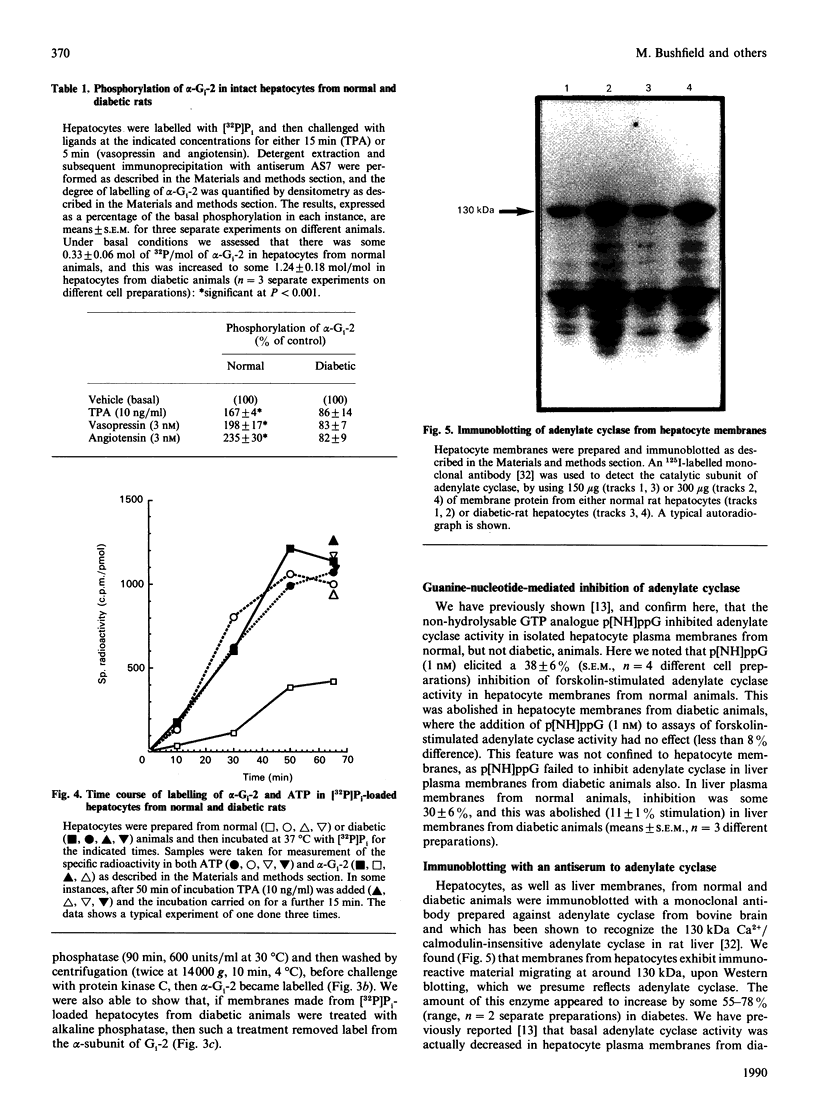


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bushfield M., Murphy G. J., Lavan B. E., Parker P. J., Hruby V. J., Milligan G., Houslay M. D. Hormonal regulation of Gi2 alpha-subunit phosphorylation in intact hepatocytes. Biochem J. 1990 Jun 1;268(2):449–457. doi: 10.1042/bj2680449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bégin-Heick N. Absence of the inhibitory effect of guanine nucleotides on adenylate cyclase activity in white adipocyte membranes of the ob/ob mouse. Effect of the ob gene. J Biol Chem. 1985 May 25;260(10):6187–6193. [PubMed] [Google Scholar]
- Bégin-Heick N. The response of adenylate cyclase to ACTH in adipocyte membranes of lean and obese mice. Mol Cell Endocrinol. 1987 Sep;53(1-2):1–8. doi: 10.1016/0303-7207(87)90185-7. [DOI] [PubMed] [Google Scholar]
- Fong H. K., Amatruda T. T., 3rd, Birren B. W., Simon M. I. Distinct forms of the beta subunit of GTP-binding regulatory proteins identified by molecular cloning. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3792–3796. doi: 10.1073/pnas.84.11.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawler D., Milligan G., Spiegel A. M., Unson C. G., Houslay M. D. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987 May 21;327(6119):229–232. doi: 10.1038/327229a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M., Qu Z. X., Moroi-Fetters S. E., Neff N. H. Apparent loss of Gi protein activity in the diabetic retina. Eur J Pharmacol. 1988 Apr 27;149(1-2):193–194. doi: 10.1016/0014-2999(88)90064-7. [DOI] [PubMed] [Google Scholar]
- Hawkins P. T., Michell R. H., Kirk C. J. A simple assay method for determination of the specific radioactivity of the gamma-phosphate group of 32P-labelled ATP. Biochem J. 1983 Mar 15;210(3):717–720. doi: 10.1042/bj2100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Hanski E., Houslay M. D. Islet-activating protein blocks glucagon desensitization in intact hepatocytes. Biochem J. 1984 Aug 15;222(1):189–194. doi: 10.1042/bj2220189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Houslay M. D. Challenge of hepatocytes by glucagon triggers a rapid modulation of adenylate cyclase activity in isolated membranes. Biochem J. 1983 Jul 15;214(1):93–98. doi: 10.1042/bj2140093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Wallace A. V., Houslay M. D. Insulin and glucagon regulate the activation of two distinct membrane-bound cyclic AMP phosphodiesterases in hepatocytes. Biochem J. 1983 Jul 15;214(1):99–110. doi: 10.1042/bj2140099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. D., Hanoune J., Birnbaumer L. Guanine nucleotide inhibition of cyc- S49 mouse lymphoma cell membrane adenylyl cyclase. J Biol Chem. 1982 Dec 25;257(24):14723–14725. [PubMed] [Google Scholar]
- Houslay M. D., Elliott K. R. Cholera toxin mediated activation of adenylate cyclase in intact rat hepatocytes. FEBS Lett. 1979 Aug 15;104(2):359–363. doi: 10.1016/0014-5793(79)80852-2. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Elliott K. R. Is the receptor-mediated endocytosis of cholera toxin A pre-requisite for its activation of adenylate cyclase in intact rat hepatocytes? FEBS Lett. 1981 Jun 15;128(2):289–292. doi: 10.1016/0014-5793(81)80101-9. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Gawler D. J., Milligan G., Wilson A. Multiple defects occur in the guanine nucleotide regulatory protein system in liver plasma membranes of obese (fa/fa) but not lean (Fa/Fa) Zucker rats: loss of functional Gi and abnormal Gs function. Cell Signal. 1989;1(1):9–22. doi: 10.1016/0898-6568(89)90016-8. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Metcalfe J. C., Warren G. B., Hesketh T. R., Smith G. A. The glucagon receptor of rat liver plasma membrane can couple to adenylate cyclase without activating it. Biochim Biophys Acta. 1976 Jun 17;436(2):489–494. doi: 10.1016/0005-2736(76)90210-8. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Wakelam M. J., Murphy G. J., Gawler D. J., Pyne N. J. Glucagon stimulates adenylate cyclase through GR2 glucagon receptors: a process which can be attenuated by glucagon stimulating inositol phospholipid metabolism through GR1 glucagon receptors. Biochem Soc Trans. 1987 Feb;15(1):21–24. doi: 10.1042/bst0150021. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Stadel J. M., Caron M. G. Adenylate cyclase-coupled beta-adrenergic receptors: structure and mechanisms of activation and desensitization. Annu Rev Biochem. 1983;52:159–186. doi: 10.1146/annurev.bi.52.070183.001111. [DOI] [PubMed] [Google Scholar]
- Lynch C. J., Blackmore P. F., Johnson E. H., Wange R. L., Krone P. K., Exton J. H. Guanine nucleotide binding regulatory proteins and adenylate cyclase in livers of streptozotocin- and BB/Wor-diabetic rats. Immunodetection of Gs and Gi with antisera prepared against synthetic peptides. J Clin Invest. 1989 Jun;83(6):2050–2062. doi: 10.1172/JCI114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchmont R. J., Ayad S. R., Houslay M. D. Purification and properties of the insulin-stimulated cyclic AMP phosphodiesterase from rat liver plasma membranes. Biochem J. 1981 Jun 1;195(3):645–652. doi: 10.1042/bj1950645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell F. M., Griffiths S. L., Saggerson E. D., Houslay M. D., Knowler J. T., Milligan G. Guanine-nucleotide-binding proteins expressed in rat white adipose tissue. Identification of both mRNAs and proteins corresponding to Gi1, Gi2 and Gi3. Biochem J. 1989 Sep 1;262(2):403–408. doi: 10.1042/bj2620403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollner S., Pfeuffer T. Two different adenylyl cyclases in brain distinguished by monoclonal antibodies. Eur J Biochem. 1988 Jan 15;171(1-2):265–271. doi: 10.1111/j.1432-1033.1988.tb13785.x. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Stabel S., Waterfield M. D. Purification to homogeneity of protein kinase C from bovine brain--identity with the phorbol ester receptor. EMBO J. 1984 May;3(5):953–959. doi: 10.1002/j.1460-2075.1984.tb01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S. R., Tsai S. C., Adamik R., Angus C. W., Serventi I. M., Tsuchiya M., Moss J., Vaughan M. Expression of Go alpha mRNA and protein in bovine tissues. Biochemistry. 1989 May 2;28(9):3803–3807. doi: 10.1021/bi00435a027. [DOI] [PubMed] [Google Scholar]
- Prpić V., Green K. C., Blackmore P. F., Exton J. H. Vasopressin-, angiotensin II-, and alpha 1-adrenergic-induced inhibition of Ca2+ transport by rat liver plasma membrane vesicles. J Biol Chem. 1984 Feb 10;259(3):1382–1385. [PubMed] [Google Scholar]
- Pyne N. J., Murphy G. J., Milligan G., Houslay M. D. Treatment of intact hepatocytes with either the phorbol ester TPA or glucagon elicits the phosphorylation and functional inactivation of the inhibitory guanine nucleotide regulatory protein Gi. FEBS Lett. 1989 Jan 16;243(1):77–82. doi: 10.1016/0014-5793(89)81221-9. [DOI] [PubMed] [Google Scholar]
- Schnabel P., Böhm M., Gierschik P., Jakobs K. H., Erdmann E. Improvement of cholera toxin-catalyzed ADP-ribosylation by endogenous ADP-ribosylation factor from bovine brain provides evidence for an unchanged amount of Gs alpha in failing human myocardium. J Mol Cell Cardiol. 1990 Jan;22(1):73–82. doi: 10.1016/0022-2828(90)90973-6. [DOI] [PubMed] [Google Scholar]
- Smith S. A., Elliott K. R., Pogson C. I. Differential effects of tryptophan on glucose synthesis in rats and guinea pigs. Biochem J. 1978 Dec 15;176(3):817–825. doi: 10.1042/bj1760817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis P. C., Northup J. K., Smigel M. D., Gilman A. G. The regulatory component of adenylate cyclase. Purification and properties. J Biol Chem. 1981 Nov 25;256(22):11517–11526. [PubMed] [Google Scholar]
- Strassheim D., Milligan G., Houslay M. D. Diabetes abolishes the GTP-dependent, but not the receptor-dependent inhibitory function of the inhibitory guanine-nucleotide-binding regulatory protein (Gi) on adipocyte adenylate cyclase activity. Biochem J. 1990 Mar 1;266(2):521–526. doi: 10.1042/bj2660521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseth T. F., Zhang H. J., Olson L. K., Schroeder W. A., Robertson R. P. Increase in Gs and cyclic AMP generation in HIT cells. Evidence that the 45-kDa alpha-subunit of Gs has greater functional activity than the 52-kDa alpha-subunit. J Biol Chem. 1989 Dec 15;264(35):21106–21111. [PubMed] [Google Scholar]
- Whetton A. D., Needham L., Dodd N. J., Heyworth C. M., Houslay M. D. Forskolin and ethanol both perturb the structure of liver plasma membranes and activate adenylate cyclase activity. Biochem Pharmacol. 1983 May 15;32(10):1601–1608. doi: 10.1016/0006-2952(83)90334-9. [DOI] [PubMed] [Google Scholar]
- Wincek T. J., Hupka A. L., Sweat F. W. Stimulation of adenylate cyclase from isolated hepatocytes and Kupffer cells. J Biol Chem. 1975 Nov 25;250(22):8863–8873. [PubMed] [Google Scholar]