Abstract
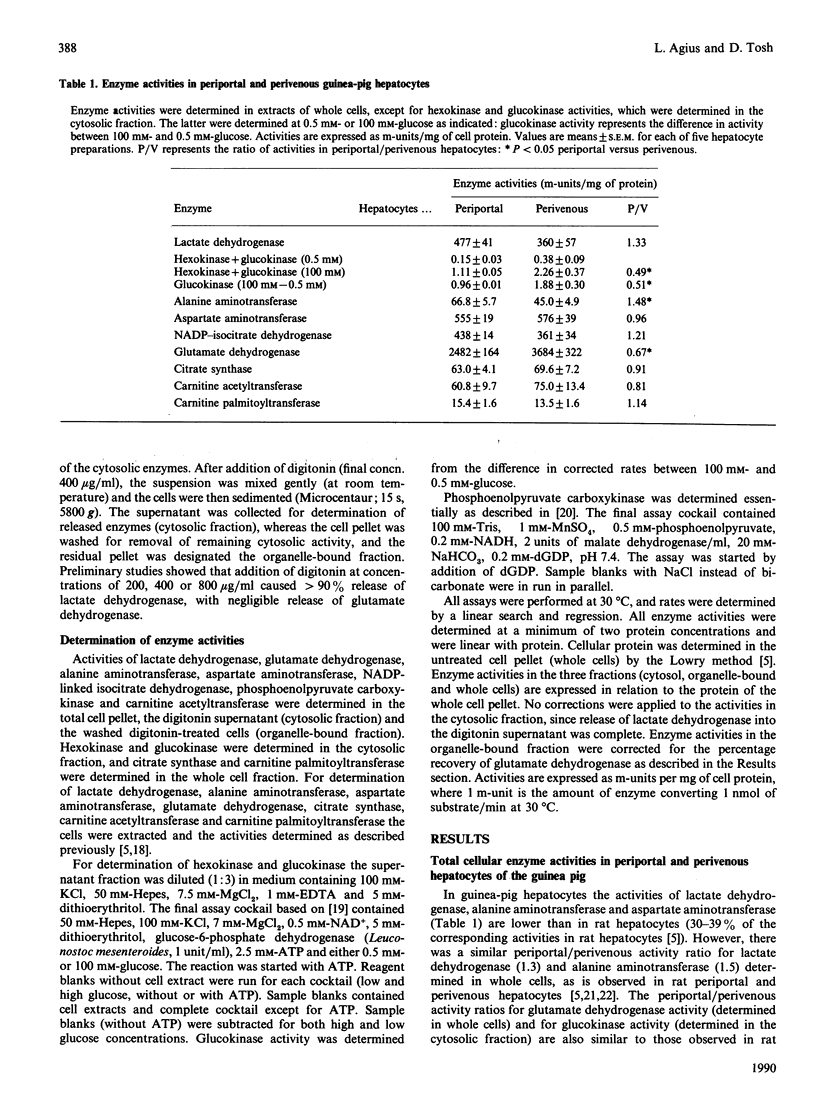
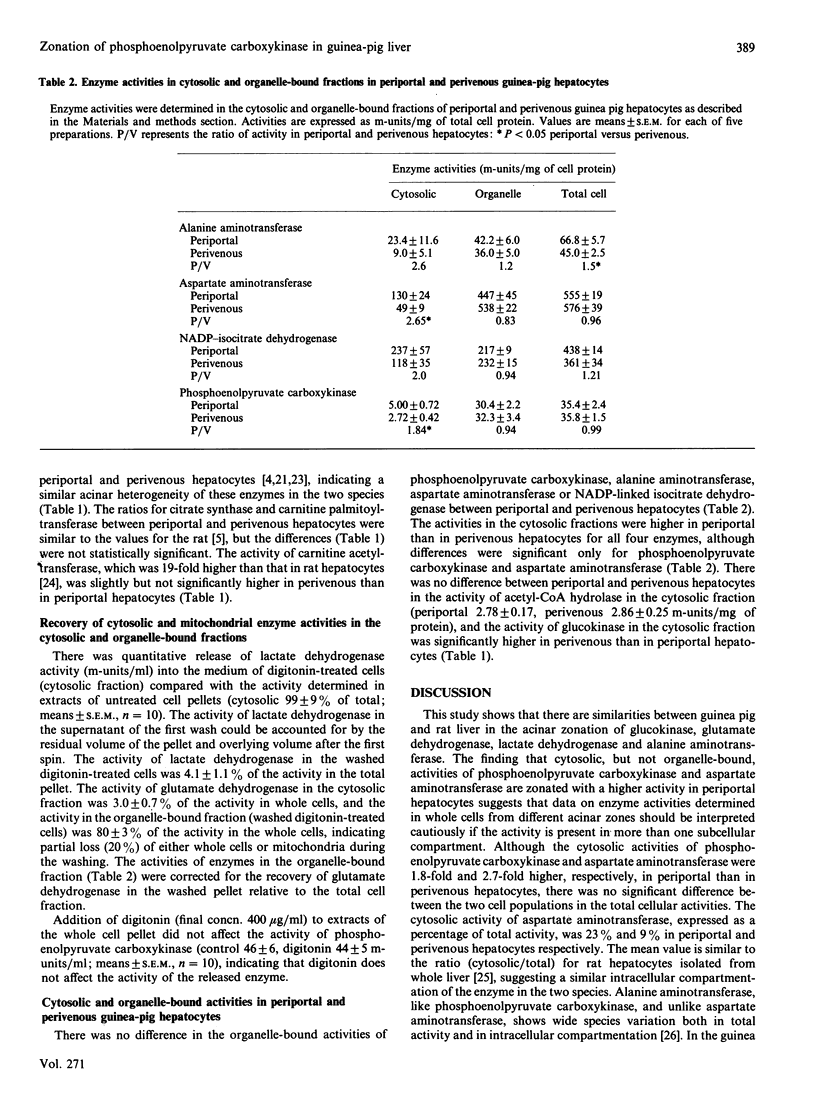
In human liver, unlike in rat liver, there is no apparent acinar heterogeneity of total cellular activity of phosphoenolpyruvate carboxykinase [Wimmer, Luttringer & Columbi (1990) Histochemistry 93, 409-415]. Since the intracellular compartmentation of phosphoenolpyruvate carbonxykinase differs in rat and human liver, we examined the acinar heterogeneity of cytosolic and organelle-bound activities of this enzyme in the guinea pig, which shows a more similar intracellular compartmentation of enzyme activity to human liver than does the rat. Cytosolic phosphoenolpyruvate carboxykinase activity was higher in periportal than in perivenous hepatocytes, whereas the organelle-bound activity was similar in the two cell populations. Aspartate aminotransferase and alanine aminotransferase activities showed a similar distribution to phosphoenolpyruvate carboxykinase, with a higher cytosolic activity in periportal than in perivenous hepatocytes but a similar organelle-bound activity in the two cell populations. Data on the acinar zonation of enzymes determined in whole cells or tissue should be interpreted cautiously if the enzyme activity is present in more than one subcellular compartment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agius L., Wright P. D., Alberti K. G. Carnitine acyltransferases and acyl-CoA hydrolases in human and rat liver. Clin Sci (Lond) 1987 Jul;73(1):3–10. doi: 10.1042/cs0730003. [DOI] [PubMed] [Google Scholar]
- Arinze I. J., Garber A. J., Hanson R. W. The regulation of gluconeogenesis in mammalian liver. The role of mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1973 Apr 10;248(7):2266–2274. [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. Purification of phosphoenolpyruvate carboxykinase from the cytosol fraction of rat liver and the immunochemical demonstration of differences between this enzyme and the mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1969 Oct 25;244(20):5625–5630. [PubMed] [Google Scholar]
- Brech W., Shrago E., Wilken D. Studies on pyruvate carboxylase in rat and human liver. Biochim Biophys Acta. 1970 Feb 24;201(2):145–154. doi: 10.1016/0304-4165(70)90288-6. [DOI] [PubMed] [Google Scholar]
- Burger H. J., Gebhardt R., Mayer C., Mecke D. Different capacities for amino acid transport in periportal and perivenous hepatocytes isolated by digitonin/collagenase perfusion. Hepatology. 1989 Jan;9(1):22–28. doi: 10.1002/hep.1840090105. [DOI] [PubMed] [Google Scholar]
- Chen K. S., Katz J. Zonation of glycogen and glucose syntheses, but not glycolysis, in rat liver. Biochem J. 1988 Oct 1;255(1):99–104. doi: 10.1042/bj2550099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell N. W., Schramm V. L., Kerich M. J., Emig F. A. Subcellular location of phosphoenolpyruvate carboxykinase in hepatocytes from fed and starved rats. J Nutr. 1986 Jun;116(6):1101–1108. doi: 10.1093/jn/116.6.1101. [DOI] [PubMed] [Google Scholar]
- Davidson A. L., Arion W. J. Factors underlying significant underestimations of glucokinase activity in crude liver extracts: physiological implications of higher cellular activity. Arch Biochem Biophys. 1987 Feb 15;253(1):156–167. doi: 10.1016/0003-9861(87)90648-5. [DOI] [PubMed] [Google Scholar]
- DeRosa G., Swick R. W. Metabolic implications of the distribution of the alanine aminotransferase isoenzymes. J Biol Chem. 1975 Oct 25;250(20):7961–7967. [PubMed] [Google Scholar]
- Elliott K. R., Pogson C. I. The effects of starvation and experimental diabetes on phosphoenol-pyruvate carboxykinase in the guinea pig. Biochem J. 1977 May 15;164(2):357–361. doi: 10.1042/bj1640357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A. J., Hanson R. W. The interrelationships of the various pathways forming gluconeogenic precursors in guinea pig liver mitochondria. J Biol Chem. 1971 Feb 10;246(3):589–598. [PubMed] [Google Scholar]
- Groen A. K., Sips H. J., Vervoorn R. C., Tager J. M. Intracellular compartmentation and control of alanine metabolism in rat liver parenchymal cells. Eur J Biochem. 1982 Feb;122(1):87–93. doi: 10.1111/j.1432-1033.1982.tb05851.x. [DOI] [PubMed] [Google Scholar]
- Gunn J. M., Ballard F. J., Hanson R. W. Infulence of hormones and medium composition on the degradation of phosphoenolpyruvate carboxykinase (GTP) and total protein in Reuber H35 cells. J Biol Chem. 1976 Jun 25;251(12):3586–3593. [PubMed] [Google Scholar]
- Guzmán M., Castro J. Zonation of fatty acid metabolism in rat liver. Biochem J. 1989 Nov 15;264(1):107–113. doi: 10.1042/bj2640107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T., Matsumoto M. Effects of nutrition and ontogeny on liver cytosolic and mitochondrial phosphoenolpyruvate carboxykinase activity of the rat, hamster, guinea-pig, pig, kid, calf and chick. Comp Biochem Physiol B. 1984;77(3):547–550. doi: 10.1016/0305-0491(84)90273-6. [DOI] [PubMed] [Google Scholar]
- Heitzman R. J., Herriman I. D., Mallinson C. B. Some effects of glucocorticoids on the subcellular distribution of the activities of citrate synthase and phosphoenolpyruvate carboxykinase in livers of rats and cows. FEBS Lett. 1972 Jan 15;20(1):19–21. doi: 10.1016/0014-5793(72)80006-1. [DOI] [PubMed] [Google Scholar]
- Horio Y., Fukui H., Taketoshi M., Tanaka T., Wada H. Induction of cytosolic aspartate aminotransferase by glucagon in primary cultured rat hepatocytes. Biochem Biophys Res Commun. 1988 May 31;153(1):410–416. doi: 10.1016/s0006-291x(88)81239-7. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989 Jul;69(3):708–764. doi: 10.1152/physrev.1989.69.3.708. [DOI] [PubMed] [Google Scholar]
- Lenartowicz E., Wojtczak A. B. Significance of the alanine aminotransferase reaction in the formation of alpha-ketoglutarate in rat liver mitochondria. Arch Biochem Biophys. 1988 Jan;260(1):309–319. doi: 10.1016/0003-9861(88)90455-9. [DOI] [PubMed] [Google Scholar]
- NORDLIE R. C., LARDY H. A. Mammalian liver phosphoneolpyruvate carboxykinase activities. J Biol Chem. 1963 Jul;238:2259–2263. [PubMed] [Google Scholar]
- Petrescu I., Bojan O., Saied M., Bârzu O., Schmidt F., Kühnle H. F. Determination of phosphoenolpyruvate carboxykinase activity with deoxyguanosine 5'-diphosphate as nucleotide substrate. Anal Biochem. 1979 Jul 15;96(2):279–281. doi: 10.1016/0003-2697(79)90582-7. [DOI] [PubMed] [Google Scholar]
- Pösö A. R., Penttilä K. E., Suolinna E. M., Lindros K. O. Urea synthesis in freshly isolated and in cultured periportal and perivenous hepatocytes. Biochem J. 1986 Oct 15;239(2):263–267. doi: 10.1042/bj2390263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistorff B. Gluconeogenesis in periportal and perivenous hepatocytes of rat liver, isolated by a new high-yield digitonin/collagenase perfusion technique. Biochem J. 1985 Jul 1;229(1):221–226. doi: 10.1042/bj2290221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson D., Evans C. J. The activities and intracellular distribution of nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase, phosphoenolpyruvate carboxykinase and pyruvate carboxylase in rat, guinea-pig and rabbit tissues. Biochem J. 1975 Feb;146(2):329–332. doi: 10.1042/bj1460329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt U., Schmid H., Guder W. G. Liver cell heterogeneity. The distribution of fructose-bisphosphatase in fed and fasted rats and in man. Hoppe Seylers Z Physiol Chem. 1978 Feb;359(2):193–198. [PubMed] [Google Scholar]
- Sokal E. M., Trivedi P., Cheeseman P., Portmann B., Mowat A. P. The application of quantitative cytochemistry to study the acinar distribution of enzymatic activities in human liver biopsy sections. J Hepatol. 1989 Jul;9(1):42–48. doi: 10.1016/0168-8278(89)90074-3. [DOI] [PubMed] [Google Scholar]
- Söling H. D., Willms B., Kleineke J., Gehlhoff M. Regulation of gluconeogenesis in the guinea pig liver. Eur J Biochem. 1970 Oct;16(2):289–302. doi: 10.1111/j.1432-1033.1970.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Tosh D., Alberti G. M., Agius L. Glucagon regulation of gluconeogenesis and ketogenesis in periportal and perivenous rat hepatocytes. Heterogeneity of hormone action and of the mitochondrial redox state. Biochem J. 1988 Nov 15;256(1):197–204. doi: 10.1042/bj2560197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh D., Alberti K. G., Agius L. Clofibrate induces carnitine acyltransferases in periportal and perivenous zones of rat liver and does not disturb the acinar zonation of gluconeogenesis. Biochim Biophys Acta. 1989 Sep 15;992(3):245–250. doi: 10.1016/0304-4165(89)90081-0. [DOI] [PubMed] [Google Scholar]
- Utter M. F., Chuang D. T. Gluconeogenesis as a compartmentalized activity. Biochem Soc Trans. 1978;6(1):11–16. doi: 10.1042/bst0060011a. [DOI] [PubMed] [Google Scholar]
- Watford M., Hod Y., Chiao Y. B., Utter M. F., Hanson R. W. The unique role of the kidney in gluconeogenesis in the chicken. The significance of a cytosolic form of phosphoenolpyruvate carboxykinase. J Biol Chem. 1981 Oct 10;256(19):10023–10027. [PubMed] [Google Scholar]
- Wieland O., Evertz-Prüsse E., Stukowski B. Distribution of pyruvate carboxylase and phosphoenol-pyruvate carboxikinase in human liver. FEBS Lett. 1968 Nov;2(1):26–28. doi: 10.1016/0014-5793(68)80091-2. [DOI] [PubMed] [Google Scholar]
- Wimmer M., Luttringer C., Colombi M. Enzyme activity patterns of phosphoenolpyruvate carboxykinase, pyruvate kinase, glucose-6-phosphate-dehydrogenase and malic enzyme in human liver. Histochemistry. 1990;93(4):409–415. doi: 10.1007/BF00315859. [DOI] [PubMed] [Google Scholar]
- Wimmer M. Sex differences of the influence of T3 on the topical distribution of phosphoenolpyruvate carboxykinase activity in the liver acinus. Histochemistry. 1989;92(2):109–113. doi: 10.1007/BF00490228. [DOI] [PubMed] [Google Scholar]


