Abstract
Purpose
This study aimed to assess the causal relationships between vitamin D levels and ocular disorders.
Methods
Independent genetic variables were obtained from genome-wide association studies (GWAS) and publicly available databases. The summary statistics for 25-hydroxyvitamin D (25(OH)D) were obtained from two large-scale GWAS studies, with sample sizes of 324,105 and 417,580 European individuals. The genetic variants of myopia, primary open angle glaucoma (POAG), anterior iridocyclitis, senile cataract, diabetic retinopathy (DR), retinal vein occlusion (RVO), wet age-related macular degeneration (WAMD) and optic neuritis were extracted from the latest release of FinnGen consortium, which contains genome data from Finnish participants. Subsequently, Mendelian randomization (MR) analyses were conducted to obtain effect estimates. Additionally, we performed multivariable MR analysis and mediation analysis to validate the results.
Results
In the discovery dataset, genetically predicted vitamin D concentration was found to be causally associated with an increased risk of WAMD, (odd ratio (OR) = 1.35, 95% confidence interval (CI) = 1.09–1.67, PIVW = 0.005). However, no causal effects of genetically predisposed vitamin D levels on the risk of most types of ocular disorders were observed. Reverse MR revealed no causal relationships between the ocular diseases and vitamin D concentrations. The MR analyses of the validation dataset yielded consistent results. Additionally, the causal effect of vitamin D levels on the risk of WAMD remained significant after adjusting for potential confounders in the multivariable MR analysis (OR = 1.86, 95% CI = 1.26–2.73, PIVW = 0.002).
Conclusion
Our MR analysis results provide robust evidence of a causal relationship between genetically predicted 25(OH)D levels and an increased risk of WAMD in European population. These findings offer important insights into the management and control of ocular disorders.
Keywords: vitamin D, 25(OH)D, ocular disorders, Mendelian randomization, causal relationship
Introduction
Vitamin D, a fat-soluble vitamin, plays a crucial role in various biological pathways, including immune regulation, cell growth, inhibition of apoptosis and anti-angiogenesis (1, 2). Additionally, vitamin D is involved in maintaining endothelial cell function and exhibits anti-inflammatory effects in multiple autoimmune diseases (3, 4). Sunlight exposure is recognized as the primary source of endogenous synthesis of vitamin D3 in individuals (5). Dietary intake and supplements also contribute to the formation of vitamin D3 and vitamin D2. Both vitamin D3 and vitamin D2 undergo hydroxylation in the liver, converting them into 25-hydroxyvitamin D (25(OH)D). The serum level of 25(OH)D, which reflects skin production, dietary intake and supplementation, is considered as a reliable biomarker of vitamin D status (6).
In recent years, the mechanisms and associated pathogenesis of vitamin D in ocular diseases have attracted increasing attention worldwide. Extensive studies have explored the relationships between vitamin D and ocular disorders, spanning from the anterior segment to fundus (7). Myopia, a refractive error with rapidly increasing prevalence over the world, has been the subject of investigations (8, 9). A cohort study conducted in Western Australia demonstrated significantly reduced vitamin D levels in myopic individuals compared to nonmyopic populations, after the adjusting for confounding factors (10). Similarly, evidence from a large-scale epidemiological study on 6-year-old children suggests that decreased serum concentrations of 25(OH)D is closely associated with higher axial length and an increased risk of myopia (11). However, it is worth noting that Li and colleagues found no association between 25(OH)D levels and myopia in Chinese children and adolescents (12).
Diabetic retinopathy (DR), the most common ocular complication of diabetes, can lead to irreversible blindness (13). Previous study had shown that serum levels of 25(OH)D were significantly elevated in individuals with DR compared to diabetes patients without ocular lesions (14). Decreased vitamin D concentrations could serve as a potential biomarker and predictive factor for DR. Furthermore, some scholars have reported a positive association between 25(OH)D levels and the severity of DR, while Alam and colleagues opposed this finding (15–17). Age-related macular degeneration (AMD), a chronic disease and growing public health burden in industrialized countries, has also been widely investigated (18). A large-scale multicenter study suggested no linear relationship between 25 (OH)D levels and early AMD or neovascular AMD (19). In addition, evidence from a randomized clinical trial manifested no effect of vitamin D3 supplementation on the incidence of AMD (20). Overall, the associations between 25(OH)D and risk of ocular disorders remain controversial, due to the inevitable confounding bias of clinical investigations.
Mendelian randomization (MR) is a statistical approach used to examine causal relationships between exposure factors and outcomes. Since genes are randomly distributed in the process of inheritance, the relationship between risk factor and outcome will not be influenced by confounding factors. Taken the randomly assigned genetic variables during pregnancy into consideration, the direction of causal association can also be evaluated. Compared to conventional clinical trials, MR analysis could provide robust and potent evidence for causal inference. Therefore, the objective of this study was to comprehensively determine the causal relationship between serum vitamin D levels and ocular diseases. The findings of this study are expected to yield fresh insights and clinical implications for prevention and treatment strategies.
Methods
Study design
We conducted a comprehensive bidirectional MR analysis to explore the causal associations between vitamin D levels and ocular disorders. The present MR analysis was performed in accordance to the principles proposed by the STROBE-MR statement (21). Ethical approval and informed consent were not requested. To ensure robust and reliable results, the MR analysis should be conducted according to three basic assumptions: (1) the genetic variants as instrument variables (IVs) are associated with exposures strongly; (2) the IVs are not related to any potential confounders; and (3) the IVs affect the outcome only via exposure factors rather than other pathways and phenotypes. To minimize population stratification bias, the databases of exposures and outcomes were both from European population.
Data for vitamin D and ocular disorders
The summary data for 25(OH)D was derived form a recently published genome-wide association study (GWAS) dataset, including phenotype, genotype and clinical demographics (22). This comprehensive large-scale study contained a total of 324,105 European individuals with self-reported fair skin, from the United Kingdom Biobank (UKB). To further identify the robustness of causality, we utilized another summary statistics for Vitamin D levels. This dataset contains 417,580 participants and body mass index (BMI) is considered as a covariate (23). The GWAS data for ocular disorders were collected from FinnGen research project, which is a public-private partnership aiming to analyze genome and health data from 500,000 Finnish biobank participants to understand disease mechanisms and predispositions (24). To ensure the reliability and completeness of the GWAS statistics, we extracted genetic variants from the latest data for various ocular diseases, including myopia (4,106 cases and 394,028 controls), primary open angle glaucoma (POAG: 8,530 cases and 391,275 controls), anterior iridocyclitis (7,152 cases and 405,029 controls), senile cataract (65,235 cases and 341,546 controls), DR (10,413 cases and 308,633 controls), retinal vein occlusion (RVO: 775 cases and 308,633 controls), wet AMD (WAMD, 5,239 cases and 273,920 controls) and optic neuritis (1,295 cases and 409,190 controls). Detailed characteristics of the included GWAS databases are summarized in Table 1.
Table 1.
Characteristics of GWAS databases.
| Trait | Resource | Year | Sample size | Data download |
|---|---|---|---|---|
| Exposure | ||||
| Vitamin D (Discovery) | Wang et al | 2023 | 324,105 participants | DOI: 10.1371/journal.pgen.1011033 |
| Vitamin D (Validation) | Revez et al | 2020 | 417,580 participants | DOI: 10.1038/s41467-020-15421-7 |
| HDL cholesterol | UK Biobank | 2020 | 403,943 participants | gwas.mrcieu.ac.uk/datasets/ieu-b-109/ |
| LDL cholesterol | UK Biobank | 2020 | 440,546 participants | gwas.mrcieu.ac.uk/datasets/ieu-b-110/ |
| Triglycerides | UK Biobank | 2020 | 441,016 participants | gwas.mrcieu.ac.uk/datasets/ieu-b-111/ |
| Type 2 diabetes | Xue et al | 2018 | 62,892 cases and 596,424 controls | DOI: 10.1038/s41467-018-04951-w |
| Hypertension | UK Biobank | 2018 | 119,731 cases and 343,202 controls | gwas.mrcieu.ac.uk/datasets/ukb-b-14057/ |
| CRP | Said et al | 2022 | 1,002,898 participants | DOI: 10.1038/s41467-022-29650-5 |
| Cigarettes per day | Liu et al | 2019 | 337,334 participants | DOI: 10.1038/s41588-018-0307-5 |
| Alcoholic drinks per week | Liu et al | 2019 | 1,232,091 participants | DOI: 10.1038/s41588-018-0307-5 |
| Outcome | ||||
| Myopia | Finngen | 2023 | 4,106 cases and 394,028 controls | r10.finngen.fi/ |
| POAG | Finngen | 2023 | 8,530 cases and 391,275 controls | r10.finngen.fi/ |
| Anterior iridocyclitis | Finngen | 2023 | 7,152 cases and 405,029 controls | r10.finngen.fi/ |
| Senile cataract | Finngen | 2023 | 65,235 cases and 341,546 controls | r10.finngen.fi/ |
| Diabetic retinopathy | Finngen | 2023 | 10,413 cases and 308,633 controls | r9.finngen.fi/ |
| Retinal vein occlusion | Finngen | 2023 | 775 cases and 308,633 controls | r9.finngen.fi/ |
| WAMD | Finngen | 2023 | 5,239 cases and 273,920 controls | r10.finngen.fi/ |
| Optic neuritis | Finngen | 2023 | 1,295 cases and 409,190 controls | r10.finngen.fi/ |
GWAS, genome-wide association study; CRP, C-reactive protein; POAG, primary open angle glaucoma; WAMD, wet age-related degeneration.
For the analysis, single nucleotide polymorphisms (SNPs) with genome-wide significance (p value <5 × 10−8), low linkage disequilibrium (r2 < 0.001) and a window size of 10,000 kb were selected when considering vitamin D levels as exposure. To investigate potential inverse relationships, we conducted bidirectional MR analysis by selecting SNPs at the genome-wide threshold of p value <5 × 10−6 when considering ocular diseases as exposures. We also performed linkage disequilibrium analysis to ensure independence among SNPs (r2 < 0.01 and clumping distance of 10,000 kb). Palindromic SNPs and SNPs with a minor allele frequency (MAF) less than 0.01 were excluded. Additionally, we used the F-statistic to evaluate the strength of the instrumental variables and avoid weak instrument bias, calculated using the equation: F = Beta2/SE2 (25). SNPs with a F-value less than 10 were further eliminated.
MR analysis and sensitivity analysis
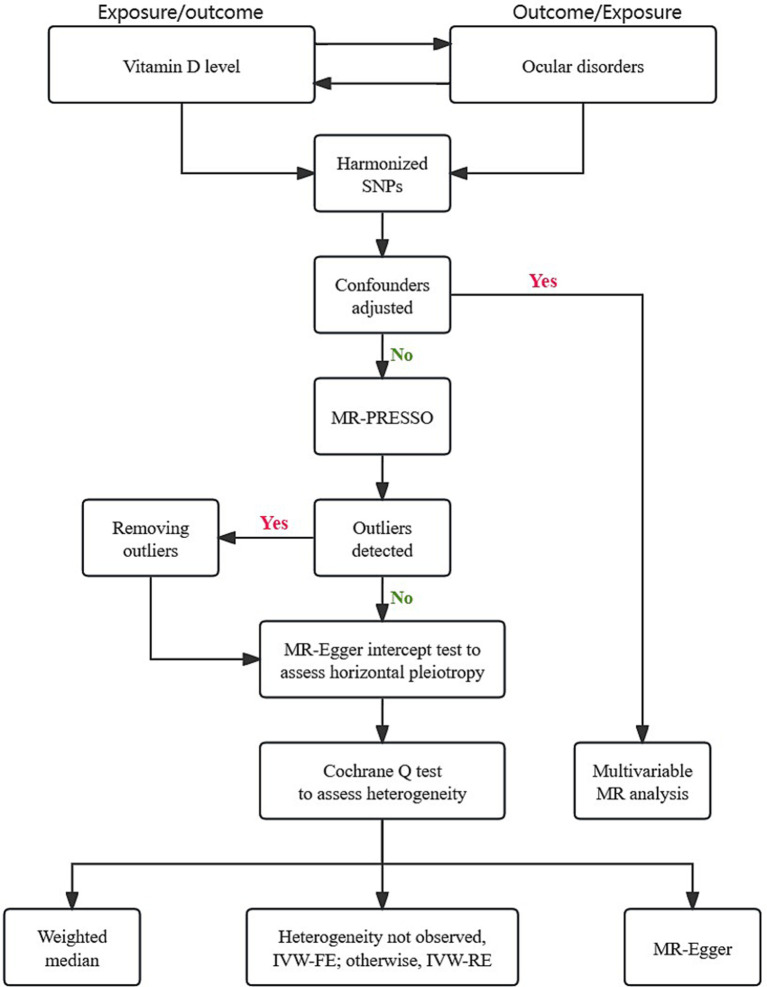
The statistical analysis was conducted as depicted in Figure 1. After harmonizing the SNPs from exposures and outcomes, we employed the MR Pleiotropy RESidual Sum and Outlier (MR-PRESSO) test to identify and correct for potential pleiotropy by eliminating outliers (26). The Cochrane Q test was employed to assess heterogeneity between different genetic variants. The fixed-effects inverse-variance weighted (IVW) model was applied as the main MR analysis approach, providing robust and unbiased estimates (27). In case of significant heterogeneity, the alternative random-effects IVW method was conducted. The weighted median (WM) approach offers accurate effects even when up to 50% genetic variables are invalid (28). The MR-Egger regression was employed to provide an unbiased effect even when the majority of genetic instrument variables are invalid (29). In addition, we utilized the intercept of the MR-Egger test to examine horizontal pleiotropy.
Figure 1.
Flow chart of overall Mendelian randomization design.
Multivariable MR (MVMR) is a novel method incorporating genetic variations for potential confounders or mediators into the same model (30). If univariable MR analysis indicated a causal relationship, MVMR analysis was employed to adjust for confounders, including type 2 diabetes, hypertension, C-reactive protein (CRP), cigarettes per day and alcoholic drinks per week (31–33). Considering the lipid-soluble nature of vitamin D, we conducted mediation analysis using two-step MR to explore the potential mediating effects of high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and triglycerides on the causal relationships between vitamin D and ocular disorders. As presented in Table 1, the summary statistics of these phenotypes were obtained from the publicly available databases and large-scale GWAS studies.
All MR analyses were carried out by R-studio software (version 4.3.1), using the “TwoSampleMR,” “MendelianRandomization” and “MRPRESSO” packages. Considering multiple tests between exposures and outcomes, a Bonferroni-corrected threshold of p < 0.05/8 was considered statistically significant.
Results
Forward MR analysis
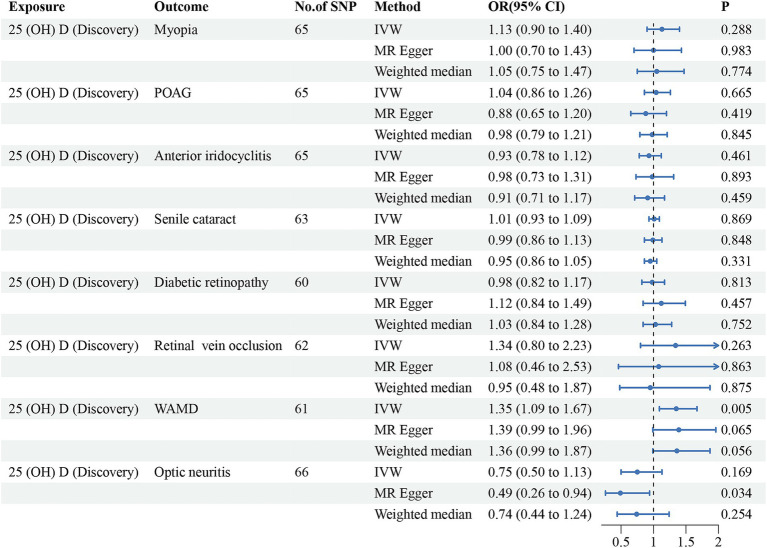
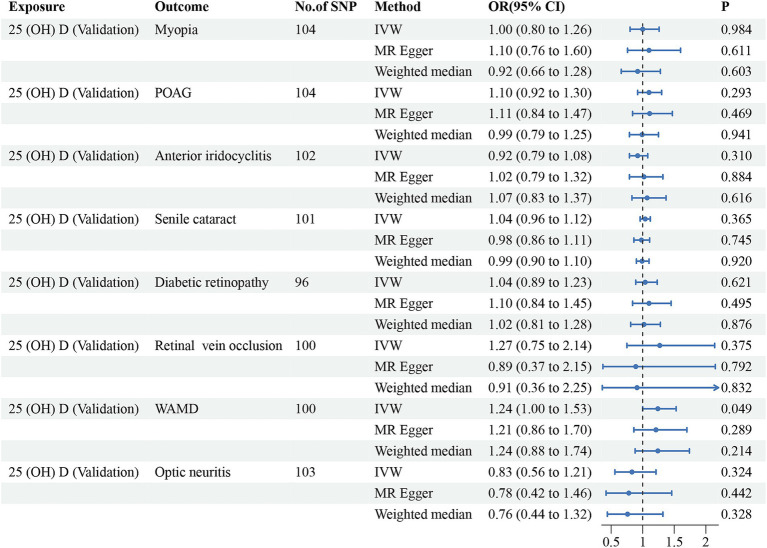
Initially, we conducted the MR-PRESSO test to identify potential pleiotropy and eliminate outliers of the discovery dataset. After eliminating the outliers, a total of 60–66 SNPs were finally included for further analysis. All of the included instrumental variants exhibited an F-statistic greater than 10, indicating their strong instrumental strength. The results, as summarized in Table 2 and Figure 2, revealed no significant associations between vitamin D levels and risk of myopia (odd ratio (OR) = 1.13, 95% confidence interval (CI) = 0.90–1.40, PIVW = 0.288), POAG (OR = 1.04, 95% CI = 0.86–1.26, PIVW = 0.665), anterior iridocyclitis (OR = 0.93, 95% CI = 0.78–1.12, PIVW = 0.461), senile cataract (OR = 1.01, 95% CI = 0.93–1.09, PIVW = 0.869), DR (OR = 0.98, 95% CI = 0.82–1.17, PIVW = 0.813), RVO (OR = 1.34, 95% CI = 0.80–2.23, PIVW = 0.263) and optic neuritis (OR = 0.75, 95% CI = 0.50–1.13, PIVW = 0.169). However, a positive association was observed between genetically predicted vitamin D concentrations and an increased risk of WAMD (OR = 1.35, 95% CI = 1.09–1.67, PIVW = 0.005). Similar trends were observed using WM and MR-Egger models, although statistical significance was not achieved. To validate these observations, we utilized another GWAS database for 25(OH)D. Outliers for anterior iridocyclitis (rs8107974), senile cataract (rs2229742) and DR (rs1260326) were identified and subsequently discarded. Employing the IVW method, a significant causal relationship was found between genetically assessed 25(OH)D levels and a higher risk of WAMD (OR = 1.24, 95% CI = 1.00–1.53, PIVW = 0.049). However, this causal association did not retain statistical significance after Bonferroni correction, suggesting potential causality. Consistently with discovery analyses, no causal relationships were identified between the remaining ocular disorders and vitamin D levels. The comprehensive results of different models were presented in Table 2 and Figure 3. The MR-Egger regression intercept test indicated the absence of horizontal pleiotropy in the forward MR analysis (Table 2). The details of SNPs, including rs number, effect allele, other allele, effect allele frequency, beta value, standard error, p-value and F-statistics were presented in Supplementary Table S1.
Table 2.
Mendelian randomization analyses between vitamin D levels and ocular disorders.
| Exposure | Outcome | IVW | Heterogeneity | Pleiotropy | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | IVW Q | p value | Intercept | p value | ||
| 25(OH)D (Discovery) | Myopia | 1.13 (0.90, 1.40) | 0.288 | 76.78 | 0.131 | 0.005 | 0.398 |
| POAG | 1.04 (0.86, 1.26) | 0.665 | 113.67 | 0.0001 | 0.007 | 0.173 | |
| AI | 0.93 (0.78, 1.12) | 0.461 | 87.28 | 0.028 | −0.002 | 0.687 | |
| SC | 1.01 (0.93, 1.09) | 0.869 | 92.53 | 0.007 | 0.0009 | 0.711 | |
| DR | 0.98 (0.82, 1.17) | 0.813 | 104.42 | 0.0002 | −0.006 | 0.267 | |
| RVO | 1.34 (0.80, 2.23) | 0.263 | 72.93 | 0.141 | 0.010 | 0.533 | |
| WAMD | 1.35 (1.09, 1.67) | 0.005* | 75.85 | 0.081 | −0.001 | 0.844 | |
| Optic neuritis | 0.75 (0.50, 1.13) | 0.169 | 83.72 | 0.059 | 0.019 | 0.103 | |
| 25(OH)D (Validation) | Myopia | 1.00 (0.80, 1.26) | 0.984 | 138.46 | 0.011 | −0.003 | 0.531 |
| POAG | 1.10 (0.92, 1.30) | 0.293 | 157.90 | 0.0004 | −0.0004 | 0.911 | |
| AI | 0.92 (0.79, 1.08) | 0.310 | 115.64 | 0.151 | −0.004 | 0.335 | |
| SC | 1.04 (0.96, 1.12) | 0.365 | 152.01 | 0.0006 | 0.002 | 0.267 | |
| DR | 1.04 (0.89, 1.23) | 0.621 | 136.92 | 0.003 | −0.002 | 0.627 | |
| RVO | 1.27 (0.75, 2.14) | 0.375 | 119.33 | 0.080 | 0.012 | 0.331 | |
| WAMD | 1.24 (1.00, 1.53) | 0.049* | 134.99 | 0.009 | 0.001 | 0.845 | |
| Optic neuritis | 0.83 (0.56, 1.21) | 0.324 | 125.29 | 0.059 | 0.002 | 0.828 | |
IVW, inverse-variance weighted; OR, odds ratio; CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D; POAG, primary open angle glaucoma; AI, anterior iridocyclitis; SC, senile cataract; DR, diabetic retinopathy; RVO, retinal vein occlusion; WAMD, wet age-related degeneration.
Figure 2.
Forest plot for the causal effects of vitamin D (discovery dataset) on the risk of ocular disorders. SNP, single nucleotide polymorphism; 25(OH)D, 25-hydroxyvitamin D; POAG, primary open angle glaucoma; WAMD, wet age-related degeneration; IVW, inverse variance weighted; 95%CI, 95% confidence interval.
Figure 3.
Forest plot for the causal estimates of vitamin D (validation dataset) on the risk of ocular disorders. SNP, single nucleotide polymorphism; 25(OH)D, 25-hydroxyvitamin D; POAG, primary open angle glaucoma; WAMD, wet age-related degeneration; IVW, inverse variance weighted; 95%CI, 95% confidence interval.
Reverse MR analysis, MVMR analysis, and mediation analysis
Moving on to the reverse MR analysis, after harmonizing and MR-PRESSO test, the detailed characteristics of genetic variants were listed in Supplementary Table S2. The F-value of each genetic variable exceeded 10, indicating the absence of weak instrument bias. To ensure the robustness and reliability of MR results, at least 5 SNPs were utilized for each exposure. In relation to the discovery and validation datasets of vitamin D, various MR methods revealed no causal links between ocular disorders and levels of 25(OH)D (Supplementary Figures S1, S2). No evidence of horizontal pleiotropy was discovered through the MR-Egger intercept, thus confirming the reliability of results obtained from the reverse analysis (Supplementary Table S3).
Furthermore, we conducted MVMR analysis to assess the robustness of the significant causal relationship between vitamin D and WAMD. This analysis aimed to evaluate the potential effects of type 2 diabetes, hypertension, CRP, cigarettes per day and alcoholic drinks per week. The estimates from the discovery MVMR analysis revealed the causal association remained significant (Table 3, OR = 1.86, 95% CI = 1.26–2.73, PIVW = 0.002). Consistent results were also observed using the WM and MR-Egger models (p < 0.05) and the MVMR-Egger intercept test demonstrated the absence of horizontal pleiotropy (Egger intercept = 0.003, p = 0.325). In the validation MVMR analysis, genetically determined 25(OH)D levels were found to be not associated with the risk of WAMD, after adjusting for potential confounders. Additionally, using the lipid parameters as mediators, no significant mediation relationship was found (Supplementary Table S4).
Table 3.
Multivariable MR on the causal associations between 25(OH)D levels and WAMD.
| Exposure | Outcome | Number of SNPs | Method | OR (95% CI) | p value |
|---|---|---|---|---|---|
| 25(OH)D (Discovery) | WAMD | 227 | IVW | 1.86 (1.26, 2.73) | 0.002 |
| 227 | MR-Egger | 1.85 (1.25, 2.73) | 0.002 | ||
| 227 | Weighted median | 1.87 (1.20, 2.92) | 0.006 | ||
| 25(OH)D (Validation) | WAMD | 238 | IVW | 1.11 (0.75, 1.65) | 0.590 |
| 238 | MR-Egger | 1.11 (0.75, 1.63) | 0.614 | ||
| 238 | Weighted median | 1.02 (0.63, 1.64) | 0.943 |
25(OH)D, 25-hydroxyvitamin D; WAMD, wet age-related degeneration; OR, odds ratio; CI, confidence interval; IVW, inverse-variance weighted.
Discussion
To the best of our knowledge, no prior studies have comprehensively explored the causal associations between vitamin D and 8 types of ocular disorders. Our fundings indicate that genetically determined 25(OH)D levels are associated with an increased risk of WAMD, which is further validated by MVMR analysis. Additionally, reverse MR analysis demonstrated no causal relationships between ocular diseases and 25(OH)D concentrations.
The most noteworthy finding emerging from this MR analysis is the causal effect of vitamin D on an increased risk of WAMD, which should be interpreted with caution. The mechanisms of AMD remain not fully understood, but there is a consensus that dysfunction of retinal pigment epithelium (RPE) plays an essential role. RPE is characterized as a pivotal component of blood retinal outer barrier, participating in the phagocytosis of photoreceptor outer segments and scavenging the damaged ROS (34). Multiple risk factors have been identified in the development of AMD, including age, smoking, genetic factors and sunlight exposure (35). Geographic position and insolation have been identified as essential factors in the prevalence of AMD, as demonstrated by meta-regression analysis (36). It has been shown that ultraviolet and blue light can lead to the damage of RPE cells (37–39). A case–control study found that sunlight exposure during working life could increase the risk of early and late AMD (40). Individuals born in summer were observed to have a higher season-specific risk of neovascular AMD compared to those born in winter (41). Additionally, a study from the United States found a peak in 25(OH)D concentrations in August and a trough in February (42). Since sunlight exposure is the main source of vitamin D, we infer that additional sunlight exposure may increase the risk of WAMD. As described in previous MR analyses, the serum lipid biomarkers, CRP levels, smoking and alcohol intake are causally associated with AMD (43–47). To account for the potential effects of these confounders, we performed the MVMR and mediation analyses. The results indicated that the causal relationship remained significant after adjusting for confounders and no mediation effects of lipid parameters were observed, further validating the robustness of causal estimates.
As widely acknowledged, cross-sectional studies cannot determine whether deficiency of vitamin D is the cause or result of diseases. In view of several unavoidable bias in clinical trials, the evidence from previous investigations for the relationships between vitamin D and risk of ocular diseases is controversial. From the perspective of genetics, we confirmed there were no causal links between vitamin D and other types of ocular disorders. A published MR analysis indicates vitamin D levels contribute little to the degree of myopia, while time spent outdoors is identified as an essential confounder (48). As previous reported, dopamine plays critical roles in the retina development and visual signaling (49). It has been suggested that time spent outdoors, resulting in sunlight exposure, prevents myopia through the dopamine-mediated pathway (50). Glaucoma, a leading cause of irreversible visual impairment, is characterized by the damage to optic nerve head and visual field (51). As previously reported, a decreased 25(OH)D concentration was associated with a higher risk of POAG (52, 53). The investigators speculate that the status of vitamin D deficiency may deteriorate the biological functions of optic nerve through oxidative stress pathway. A meta-analysis has manifested no significant difference in serum vitamin D levels between glaucoma patients and healthy controls, which is consistent with our genetic results (54). Further work is required to investigate the detailed mechanisms of vitamin D in various subtypes of glaucoma.
Prior meta-analyses have revealed positive correlations between vitamin D deficiency and a higher risk of non-infectious uveitis (55). Vitamin D has been demonstrated to paly vital roles in immune responses and gut microbiota composition (56). The deficiency of vitamin D may lead to immune dysregulation and imbalance of gut microbiota, potentially activating and accelerating the progression of uveitis. However, the majority of included studies in meta-analysis are designed as case–control and cross-sectional, limiting the robustness of results. Additionally, a two-sample MR analysis indicates positive causal effects of low vitamin D levels on the risk of uveitis (57). The GWAS information used in the present MR analysis was extracted from anterior iridocyclitis patients, so the discrepancy of MR results could be attribute to variations in characteristics among different GWAS databases. To elucidate the pathogenesis of vitamin D in cataract, a random controlled trial was performed in 23,315 older Australian adults (58). The authors found that high-dose routine vitamin D supplementation had no effect on the need for cataract surgery, which is in accordance with our MR results.
Retrospective studies reported no significant relationship between vitamin D status and DR after controlling for confounding factors (17, 59). The authors also found that majority of DR patients were lack of vitamin D, the association between degree of retinopathy and vitamin D could not be fully investigated. Regarding the RVO and optic neuritis, there remains a paucity of valid evidence. A case–control study showed no differences in vitamin D levels between central RVO patients and healthy controls (60). In addition, data from a randomized and placebo-controlled trial demonstrated that oral supplementation of vitamin D had no significant effect on the thickness of retinal nerve fiber layer in optic neuritis patients (61). It is worthwhile to point out that the sample sizes of these studies are relatively small, longitudinal and prospective trials are warranted to elucidate our results in the future.
Some strengthens of this MR analysis are worth noting. First, the MR design ensures the strengthen of genetic variables, reducing the influence of potential confounders in observational studies and providing a robust estimate of causal associations. Second, the relatively large sample size in the MR analysis allows for reliable and accurate causal relationships. However, several certain limitations should be considered. First, the genetic instruments extracted from GWAS data are from European individuals, the results of this study may not be explainable in other populations and races. Additionally, despite employing various approaches to evaluate and adjust for possible confounding and pleiotropic effects, potential confounding factors could not be completely ruled out. Taken the complex biological pathways of Vitamin D into consideration, the results of MR analysis were acquired via the genetic pathway, which may only represent a specific perspective. Moreover, due to the limited demographics and characteristics of the GWAS data, performing subgroup analyses seemed challenging, limiting the comprehensiveness of the present MR analysis.
In conclusion, genetically predicted vitamin D was causally associated with an enhanced risk of WAMD in the European population. Additionally, based on the current MR analysis, we found no convincing evidence of causal links between vitamin D concentrations and most types of ocular disorders. Our results provide novel insights into the prevention and management of ocular diseases. Further randomized controlled trials are needed to evaluate and confirm our findings in the future.
Acknowledgments
We want to acknowledge the participants and investigators of the included GWAS databases. The authors thank these researchers for their selfless sharing.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. We would like to thank all of the donors that participated in the present study. This study was supported by the Lishui Municipal Science and Technology Project (Grant no. 2023GYX66), Youth Fund Program of Lishui Municipal Central Hospital (Grant nos. 2022qnjj15 and 2022qnjj10), and Health Development Planning Commission Science Foundation of Zhejiang Province (Grant no. 2020364455).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
SF: Conceptualization, Writing – original draft, Writing – review & editing. X-yS: Conceptualization, Investigation, Writing – original draft. XL: Formal analysis, Writing – review & editing. JL: Writing – review & editing. S-pY: Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1431170/full#supplementary-material
References
- 1.Rak K, Bronkowska M. Immunomodulatory effect of vitamin D and its potential role in the prevention and treatment of type 1 diabetes mellitus-a narrative review. Molecules. (2018) 24:53. doi: 10.3390/molecules24010053, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nebbioso M, Buomprisco G, Pascarella A, Pescosolido N. Modulatory effects of 1,25-dihydroxyvitamin D3 on eye disorders: a critical review. Crit Rev Food Sci Nutr. (2017) 57:559–65. doi: 10.1080/10408398.2014.893504, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Mangin M, Sinha R, Fincher K. Inflammation and vitamin D: the infection connection. Inflamm Res. (2014) 63:803–19. doi: 10.1007/s00011-014-0755-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussin AM, Ashor AW, Schoenmakers I, Hill T, Mathers JC, Siervo M. Effects of vitamin D supplementation on endothelial function: a systematic review and meta-analysis of randomised clinical trials. Eur J Nutr. (2017) 56:1095–104. doi: 10.1007/s00394-016-1159-3 [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. (2004) 79:362–71. doi: 10.1093/ajcn/79.3.362, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Cashman KD, Van Den Heuvel EG, Schoemaker RJ, Prévéraud DP, Macdonald HM, Arcot J. 25-Hydroxyvitamin D as a biomarker of vitamin D status and its modeling to inform strategies for prevention of vitamin D deficiency within the population. Adv Nutr. (2017) 8:947–57. doi: 10.3945/an.117.015578, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan HN, Zhang XJ, Ling XT, Bui CH, Wang YM, Ip P, et al. Vitamin D and ocular diseases: a systematic review. Int J Mol Sci. (2022) 23:4226. doi: 10.3390/ijms23084226, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.French AN, Morgan IG, Burlutsky G, Mitchell P, Rose KA. Prevalence and 5- to 6-year incidence and progression of myopia and hyperopia in Australian schoolchildren. Ophthalmology. (2013) 120:1482–91. doi: 10.1016/j.ophtha.2012.12.018, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Ma Y, Qu X, Zhu X, Xu X, Zhu J, Sankaridurg P, et al. Age-specific prevalence of visual impairment and refractive error in children aged 3-10 years in Shanghai, China. Invest Ophthalmol Vis Sci. (2016) 57:6188–96. doi: 10.1167/iovs.16-20243, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Yazar S, Hewitt AW, Black LJ, Mcknight CM, Mountain JA, Sherwin JC, et al. Myopia is associated with lower vitamin D status in young adults. Invest Ophthalmol Vis Sci. (2014) 55:4552–9. doi: 10.1167/iovs.14-14589 [DOI] [PubMed] [Google Scholar]
- 11.Tideman JW, Polling JR, Voortman T, Jaddoe VW, Uitterlinden AG, Hofman A, et al. Low serum vitamin D is associated with axial length and risk of myopia in young children. Eur J Epidemiol. (2016) 31:491–9. doi: 10.1007/s10654-016-0128-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Lin H, Jiang L, Chen X, Chen J, Lu F. Low serum vitamin D is not correlated with myopia in Chinese children and adolescents. Front Med (Lausanne). (2022) 9:809787. doi: 10.3389/fmed.2022.809787, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusuhara S, Fukushima Y, Ogura S, Inoue N, Uemura A. Pathophysiology of diabetic retinopathy: the old and the new. Diabetes Metab J. (2018) 42:364–76. doi: 10.4093/dmj.2018.0182, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutlu U, Ikram MA, Hofman A, De Jong P, Uitterlinden AG, Klaver CCW, et al. Vitamin D and retinal microvascular damage: the Rotterdam study. Medicine (Baltimore). (2016) 95:e5477. doi: 10.1097/md.0000000000005477, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patrick PA, Visintainer PF, Shi Q, Weiss IA, Brand DA. Vitamin D and retinopathy in adults with diabetes mellitus. Arch Ophthalmol. (2012) 130:756–60. doi: 10.1001/archophthalmol.2011.2749 [DOI] [PubMed] [Google Scholar]
- 16.Alcubierre N, Valls J, Rubinat E, Cao G, Esquerda A, Traveset A, et al. Vitamin D deficiency is associated with the presence and severity of diabetic retinopathy in type 2 diabetes mellitus. J Diabetes Res. (2015) 2015:374178: 1–7. doi: 10.1155/2015/374178, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam U, Amjad Y, Chan AW, Asghar O, Petropoulos IN, Malik RA. Vitamin D deficiency is not associated with diabetic retinopathy or maculopathy. J Diabetes Res. (2016) 2016:1–7. doi: 10.1155/2016/6156217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guymer RH, Campbell TG. Age-related macular degeneration. Lancet. (2023) 401:1459–72. doi: 10.1016/s0140-6736(22)02609-5 [DOI] [PubMed] [Google Scholar]
- 19.Mckay GJ, Young IS, Mcginty A, Bentham GC, Chakravarthy U, Rahu M, et al. Associations between serum vitamin D and genetic variants in vitamin D pathways and age-related macular degeneration in the European eye study. Ophthalmology. (2017) 124:90–6. doi: 10.1016/j.ophtha.2016.09.007, PMID: [DOI] [PubMed] [Google Scholar]
- 20.Christen WG, Cook NR, Manson JE, Buring JE, Chasman DI, Lee IM, et al. Effect of vitamin D and ω-3 fatty acid supplementation on risk of age-related macular degeneration: An ancillary study of the VITAL randomized clinical trial. JAMA Ophthalmol. (2020) 138:1280–9. doi: 10.1001/jamaophthalmol.2020.4409, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skrivankova VW, Richmond RC, BaR W, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236, PMID: [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Hivert V, Groot S, Wang Y, Yengo L, Mcgrath JJ, et al. Cross-ancestry analyses identify new genetic loci associated with 25-hydroxyvitamin D. PLoS Genet. (2023) 19:e1011033. doi: 10.1371/journal.pgen.1011033, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Revez JA, Lin T, Qiao Z, Xue A, Holtz Y, Zhu Z, et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat Commun. (2020) 11:1647. doi: 10.1038/s41467-020-15421-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-egger regression: the role of the I2 statistic. Int J Epidemiol. (2016) 45:dyw220–dyw1974. doi: 10.1093/ije/dyw220, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. (2015) 30:543–52. doi: 10.1007/s10654-015-0011-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. (2019) 48:713–27. doi: 10.1093/ije/dyy262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue A, Wu Y, Zhu Z, Zhang F, Kemper KE, Zheng Z, et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun. (2018) 9:2941. doi: 10.1038/s41467-018-04951-w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Said S, Pazoki R, Karhunen V, Võsa U, Ligthart S, Bodinier B, et al. Genetic analysis of over half a million people characterises C-reactive protein loci. Nat Commun. (2022) 13:2198. doi: 10.1038/s41467-022-29650-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. (2019) 51:237–44. doi: 10.1038/s41588-018-0307-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somasundaran S, Constable IJ, Mellough CB, Carvalho LS. Retinal pigment epithelium and age-related macular degeneration: a review of major disease mechanisms. Clin Experiment Ophthalmol. (2020) 48:1043–56. doi: 10.1111/ceo.13834, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleckenstein M, Keenan TDL, Guymer RH, Chakravarthy U, Schmitz-Valckenberg S, Klaver CC, et al. Age-related macular degeneration. Nat Rev Dis Primers. (2021) 7:31. doi: 10.1038/s41572-021-00265-2 [DOI] [PubMed] [Google Scholar]
- 36.Reibaldi M, Longo A, Pulvirenti A, Avitabile T, Russo A, Cillino S, et al. Geo-epidemiology of age-related macular degeneration: new clues into the pathogenesis. Am J Ophthalmol. (2016) 161:e71-72: 78–93. doi: 10.1016/j.ajo.2015.09.031, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Arnault E, Barrau C, Nanteau C, Gondouin P, Bigot K, Viénot F, et al. Phototoxic action spectrum on a retinal pigment epithelium model of age-related macular degeneration exposed to sunlight normalized conditions. PLoS One. (2013) 8:e71398. doi: 10.1371/journal.pone.0071398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youn HY, Bantseev V, Bols NC, Cullen AP, Sivak JG. In vitro assays for evaluating the ultraviolet B-induced damage in cultured human retinal pigment epithelial cells. J Photochem Photobiol B. (2007) 88:21–8. doi: 10.1016/j.jphotobiol.2007.04.012, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Youn HY, McCanna D, Sivak JG, Jones LW. In vitro ultraviolet-induced damage in human corneal, lens, and retinal pigment epithelial cells. Mol Vis. (2011) 17:237–46. PMID: [PMC free article] [PubMed] [Google Scholar]
- 40.Schick T, Ersoy L, Lechanteur YT, Saksens NT, Hoyng CB, Den Hollander AI, et al. History of sunlight exposure is a risk factor for age-related macular degeneration. Retina. (2016) 36:787–90. doi: 10.1097/iae.0000000000000756, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Longo A, Casuccio A, Pani L, Avitabile T, Cillino S, Uva MG, et al. Association of neovascular age-related macular degeneration with month and season of birth in Italy. Aging (Albany NY). (2016) 9:133–41. doi: 10.18632/aging.101137, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasahara AK, Singh RJ, Noymer A. Vitamin D (25OHD) serum seasonality in the United States. PLoS One. (2013) 8:e65785. doi: 10.1371/journal.pone.0065785, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han X, Ong JS, Hewitt AW, Gharahkhani P, Macgregor S. The effects of eight serum lipid biomarkers on age-related macular degeneration risk: a Mendelian randomization study. Int J Epidemiol. (2021) 50:325–36. doi: 10.1093/ije/dyaa178, PMID: [DOI] [PubMed] [Google Scholar]
- 44.Julian TH, Cooper-Knock J, Macgregor S, Guo H, Aslam T, Sanderson E, et al. Phenome-wide Mendelian randomisation analysis identifies causal factors for age-related macular degeneration. eLife. (2023) 12:e82546. doi: 10.7554/eLife.82546, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuan V, Warwick A, Hingorani A, Tufail A, Cipriani V, Burgess S, et al. Association of Smoking, alcohol consumption, blood pressure, body mass index, and glycemic risk factors with age-related macular degeneration: a Mendelian randomization study. JAMA Ophthalmol. (2021) 139:1299–306. doi: 10.1001/jamaophthalmol.2021.4601, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han X, Ong JS, An J, Hewitt AW, Gharahkhani P, Macgregor S. Using Mendelian randomization to evaluate the causal relationship between serum C-reactive protein levels and age-related macular degeneration. Eur J Epidemiol. (2020) 35:139–46. doi: 10.1007/s10654-019-00598-z, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Yoon BW, Lee Y, Seo JH. Potential causal association between C-reactive protein levels in age-related macular degeneration: a two-sample Mendelian randomization study. Biomedicines. (2024) 12:807. doi: 10.3390/biomedicines12040807, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cuellar-Partida G, Williams KM, Yazar S, Guggenheim JA, Hewitt AW, Williams C, et al. Genetically low vitamin D concentrations and myopic refractive error: a Mendelian randomization study. Int J Epidemiol. (2017) 46:1882–90. doi: 10.1093/ije/dyx068, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou X, Pardue MT, Iuvone PM, Qu J. Dopamine signaling and myopia development: what are the key challenges. Prog Retin Eye Res. (2017) 61:60–71. doi: 10.1016/j.preteyeres.2017.06.003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang RH, Yang Q, Dong L, Li YF, Zhou WD, Wu HT, et al. Association between vitamin D and myopia in adolescents and young adults: evidence of national cross-sectional study. Eur J Ophthalmol. (2023) 33:1883–91. doi: 10.1177/11206721231161498, PMID: [DOI] [PubMed] [Google Scholar]
- 51.Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. (2017) 390:2183–93. doi: 10.1016/s0140-6736(17)31469-1 [DOI] [PubMed] [Google Scholar]
- 52.Goncalves A, Milea D, Gohier P, Jallet G, Leruez S, Baskaran M, et al. Serum vitamin D status is associated with the presence but not the severity of primary open angle glaucoma. Maturitas. (2015) 81:470–4. doi: 10.1016/j.maturitas.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 53.Yoo TK, Oh E, Hong S. Is vitamin D status associated with open-angle glaucoma? A cross-sectional study from South Korea. Public Health Nutr. (2014) 17:833–43. doi: 10.1017/s1368980013003492, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li S, Li D, Shao M, Cao W, Sun X. Lack of association between serum vitamin B₆, vitamin B(12), and vitamin D levels with different types of Glaucoma: a systematic review and Meta-analysis. Nutrients. (2017) 9:636. doi: 10.3390/nu9060636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rojas-Carabali W, Pineda-Sierra JS, Cifuentes-González C, Morales MS, Muñoz-Vargas PT, Peña-Pulgar LF, et al. Vitamin D deficiency and non-infectious uveitis: a systematic review and Meta-analysis. Autoimmun Rev. (2023) 23:103497. doi: 10.1016/j.autrev.2023.103497 [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto EA, Jørgensen TN. Relationships between vitamin D, gut microbiome, and systemic autoimmunity. Front Immunol. (2019) 10:3141. doi: 10.3389/fimmu.2019.03141, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Susarla G, Chan W, Li A, Davoudi S, Ahmadi T, Sathe S, et al. Mendelian randomization shows a causal effect of low vitamin D on non-infectious uveitis and Scleritis risk. Am J Ophthalmol. (2022) 244:11–8. doi: 10.1016/j.ajo.2022.08.001, PMID: [DOI] [PubMed] [Google Scholar]
- 58.Rahman ST, Waterhouse M, Romero BD, Baxter C, English D, Mackey DA, et al. Vitamin D supplementation and the incidence of cataract surgery in older Australian adults. Ophthalmology. (2023) 130:313–23. doi: 10.1016/j.ophtha.2022.09.015, PMID: [DOI] [PubMed] [Google Scholar]
- 59.Seyyar SA, Tıskaoğlu NS, Onder Tokuc E, Mercanlı M, Doğan L. Is serum vitamin D associated with diabetic retinopathy and its severity or with diabetes itself? Clin Exp Optom. (2023) 106:612–8. doi: 10.1080/08164622.2022.2090232, PMID: [DOI] [PubMed] [Google Scholar]
- 60.Epstein D, Kvanta A, Lindqvist PG. Vitamin D deficiency in patients with central retinal vein occlusion: a case control study. Curr Eye Res. (2017) 42:448–51. doi: 10.1080/02713683.2016.1188117 [DOI] [PubMed] [Google Scholar]
- 61.Salari M, Janghorbani M, Etemadifar M, Dehghani A, Razmjoo H, Naderian G. Effects of vitamin D on retinal nerve fiber layer in vitamin D deficient patients with optic neuritis: preliminary findings of a randomized, placebo-controlled trial. J Res Med Sci. (2015) 20:372–8. doi: 10.4103/1735-1995.158266 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.