Abstract
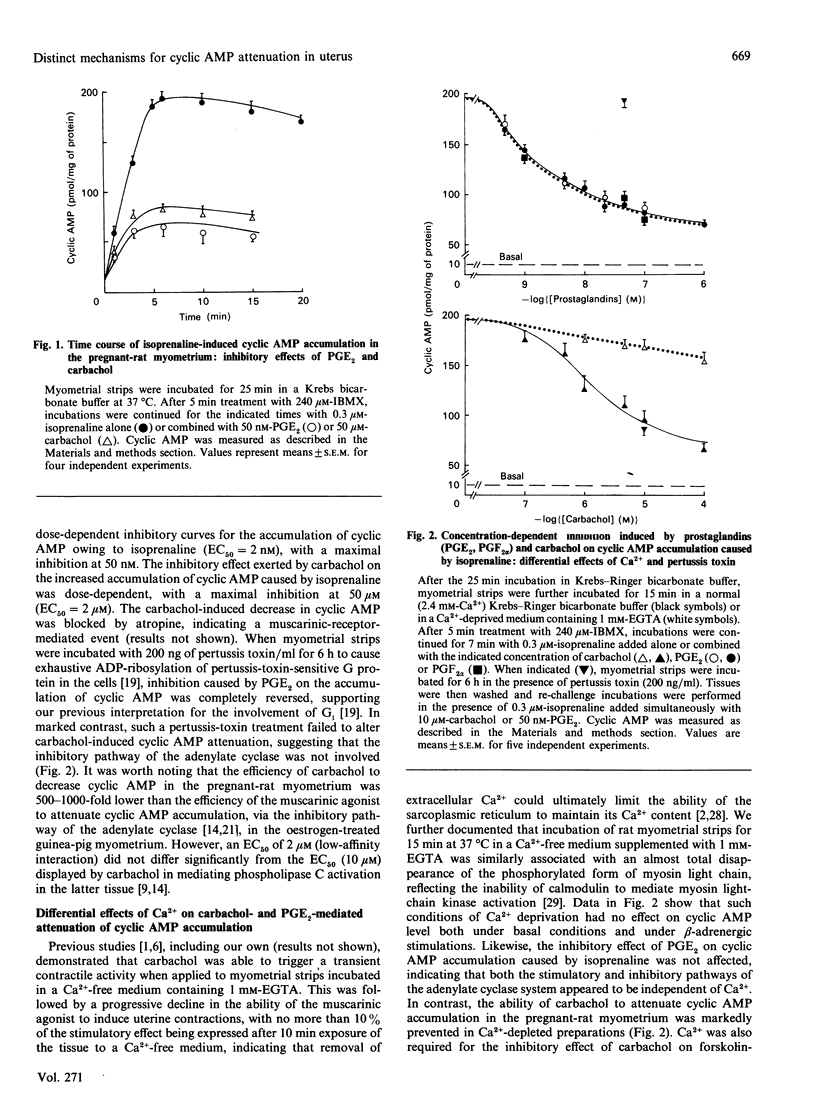
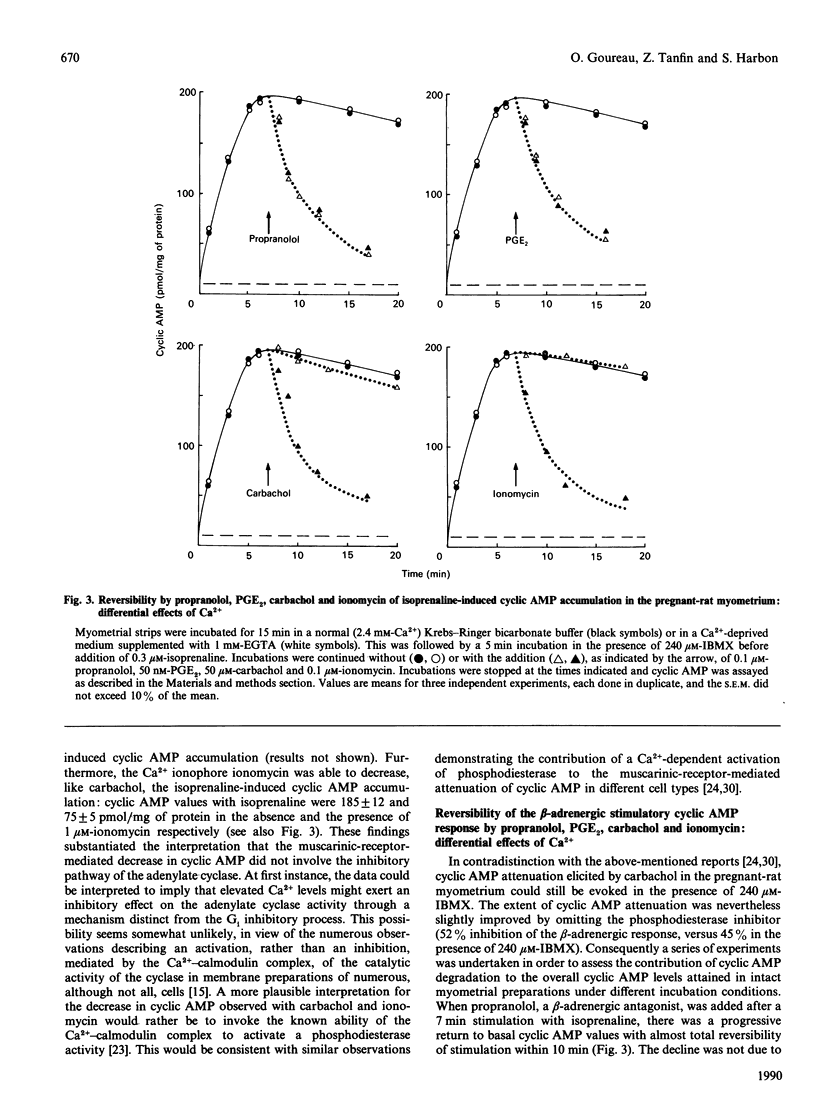
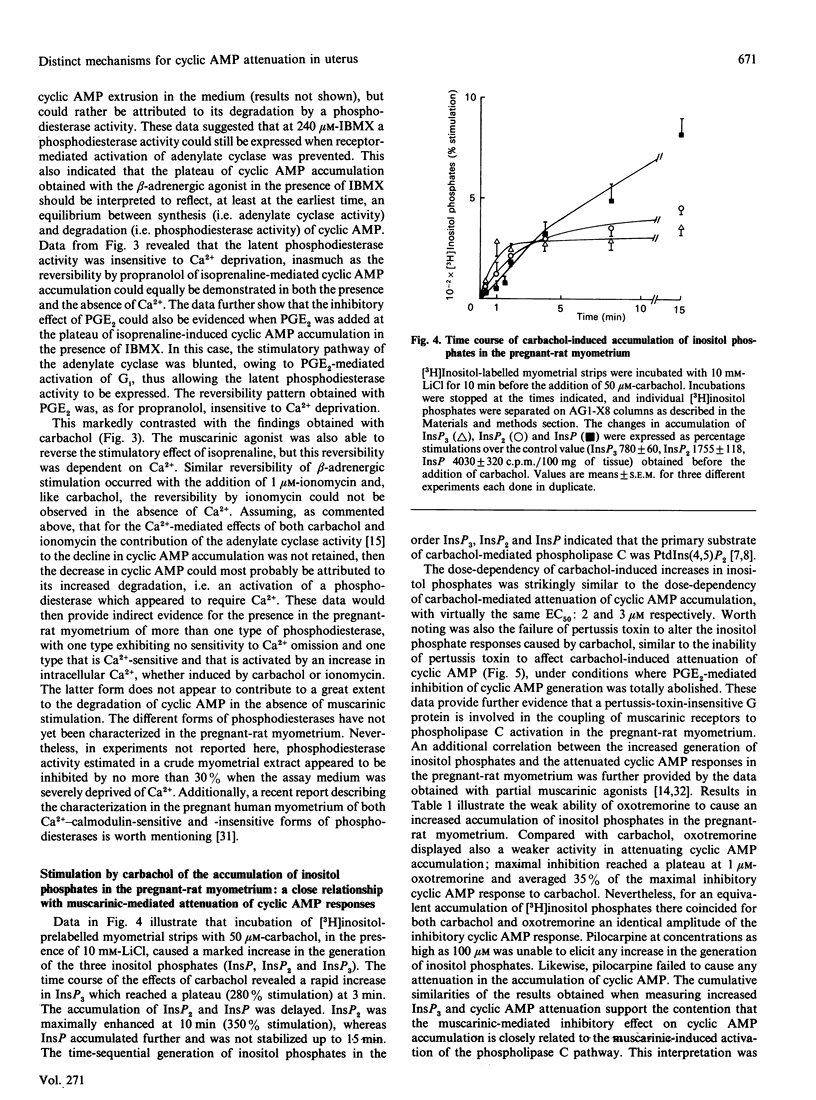
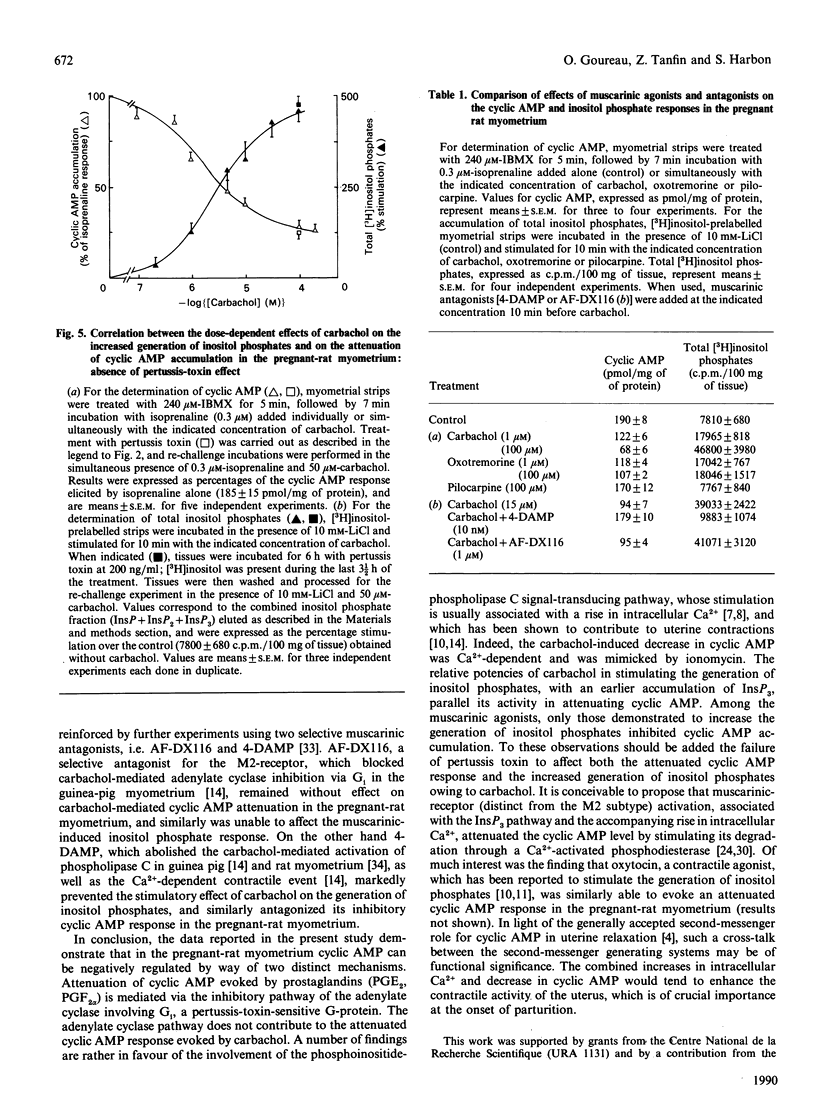
In pregnant-rat myometrium (day 21 of gestation), isoprenaline-induced cyclic AMP accumulation, resulting from receptor-mediated activation of adenylate cyclase, was negatively regulated by prostaglandins [PGE2, PGF2 alpha; EC50 (concn. giving 50% of maximal response) = 2 nM] and by the muscarinic agonist carbachol (EC50 = 2 microM). PG-induced inhibition was prevented by pertussis-toxin treatment, supporting the idea that it was mediated by the inhibitory G-protein Gi through the inhibitory pathway of the adenylate cyclase. Both isoprenaline-induced stimulation and PG-evoked inhibition of cyclic AMP were insensitive to Ca2+ depletion. By contrast, carbachol-evoked attenuation of cyclic AMP accumulation was dependent on Ca2+ and was insensitive to pertussis toxin. The inhibitory effect of carbachol was mimicked by ionomycin. Indirect evidence was thus provided for the enhancement of cyclic AMP degradation by a Ca2(+)-dependent phosphodiesterase activity in the muscarinic-mediated effect. The attenuation of cyclic AMP elicited by carbachol coincided with carbachol-stimulated inositol phosphate (InsP3, InsP2 and InsP) generation, which displayed an almost identical EC50 (3 microM) and was similarly unaffected by pertussis toxin. Both carbachol effects were reproduced by oxotremorine, whereas pilocarpine (a partial muscarinic agonist) failed to induce any decrease in cyclic AMP accumulation and concurrently was unable to stimulate the generation of inositol phosphates. These data support our proposal for a carbachol-mediated enhancement of a Ca2(+)-dependent phosphodiesterase activity, compatible with the rises in Ca2+ associated with muscarinic-induced increased generation of inositol phosphates. They further illustrate that a cross-talk between the two major transmembrane signalling systems contributed to an ultimate decrease in cyclic AMP in the pregnant-rat myometrium near term.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwer K., Hovington J. A., Sanborn B. M. Antagonism of contractants and relaxants at the level of intracellular calcium and phosphoinositide turnover in the rat uterus. Endocrinology. 1989 Jun;124(6):2995–3002. doi: 10.1210/endo-124-6-2995. [DOI] [PubMed] [Google Scholar]
- Barlow R. B., Shepherd M. K. A further search for selective antagonists at M2-muscarinic receptors. Br J Pharmacol. 1986 Dec;89(4):837–843. doi: 10.1111/j.1476-5381.1986.tb11189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Brown S. L. Agonists differentiate muscarinic receptors that inhibit cyclic AMP formation from those that stimulate phosphoinositide metabolism. J Biol Chem. 1984 Mar 25;259(6):3777–3781. [PubMed] [Google Scholar]
- Carsten M. E., Miller J. D. Ca2+ release by inositol trisphosphate from Ca2+-transporting microsomes derived from uterine sarcoplasmic reticulum. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1027–1031. doi: 10.1016/0006-291x(85)91718-8. [DOI] [PubMed] [Google Scholar]
- Do Khac L., Mokhtari A., Harbon S. A re-evaluated role for cyclic AMP in uterine relaxation. Differential effect of isoproterenol and forskolin. J Pharmacol Exp Ther. 1986 Oct;239(1):236–242. [PubMed] [Google Scholar]
- Dokhac L., D'Albis A., Janmot C., Harbon S. Myosin light chain phosphorylation in intact rat uterine smooth muscle. Role of calcium and cyclic AMP. J Muscle Res Cell Motil. 1986 Jun;7(3):259–268. doi: 10.1007/BF01753559. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbon S., Tanfin Z., Leiber D., Vesin M. F., DoKhac L. Selective interactions of cyclooxygenase and lipoxygenase metabolites with the cyclic GMP systems in the myometrium. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:639–649. [PubMed] [Google Scholar]
- Himpens B., Somlyo A. P. Free-calcium and force transients during depolarization and pharmacomechanical coupling in guinea-pig smooth muscle. J Physiol. 1988 Jan;395:507–530. doi: 10.1113/jphysiol.1988.sp016932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi H. Changes in the mechanical properties of the longitudinal and circular muscle tissues of the rat myometrium during gestation. Br J Pharmacol. 1985 Sep;86(1):247–257. doi: 10.1111/j.1476-5381.1985.tb09456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmura Y., Missiaen L., Casteels R. Properties of intracellular calcium stores in pregnant rat myometrium. Br J Pharmacol. 1988 Sep;95(1):284–290. doi: 10.1111/j.1476-5381.1988.tb16575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Ito Y., Suzuki H., Kitamura K., Itoh T. Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am J Physiol. 1982 Nov;243(5):H641–H662. doi: 10.1152/ajpheart.1982.243.5.H641. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lalanne C., Mironneau C., Mironneau J., Savineau J. P. Contractions of rat uterine smooth muscle induced by acetylcholine and angiotensin II in Ca2+-free medium. Br J Pharmacol. 1984 Feb;81(2):317–326. doi: 10.1111/j.1476-5381.1984.tb10081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiber D., Marc S., Harbon S. Pharmacological evidence for distinct muscarinic receptor subtypes coupled to the inhibition of adenylate cyclase and to the increased generation of inositol phosphates in the guinea pig myometrium. J Pharmacol Exp Ther. 1990 Feb;252(2):800–809. [PubMed] [Google Scholar]
- Leroy M. J., Cedrin I., Breuiller M., Giovagrandi Y., Ferre F. Correlation between selective inhibition of the cyclic nucleotide phosphodiesterases and the contractile activity in human pregnant myometrium near term. Biochem Pharmacol. 1989 Jan 1;38(1):9–15. doi: 10.1016/0006-2952(89)90142-1. [DOI] [PubMed] [Google Scholar]
- Marc S., Leiber D., Harbon S. Carbachol and oxytocin stimulate the generation of inositol phosphates in the guinea pig myometrium. FEBS Lett. 1986 May 26;201(1):9–14. doi: 10.1016/0014-5793(86)80561-0. [DOI] [PubMed] [Google Scholar]
- Marc S., Leiber D., Harbon S. Fluoroaluminates mimic muscarinic- and oxytocin-receptor-mediated generation of inositol phosphates and contraction in the intact guinea-pig myometrium. Role for a pertussis/cholera-toxin-insensitive G protein. Biochem J. 1988 Oct 15;255(2):705–713. [PMC free article] [PubMed] [Google Scholar]
- Meeker R. B., Harden T. K. Muscarinic cholinergic receptor-mediated activation of phosphodiesterase. Mol Pharmacol. 1982 Sep;22(2):310–319. [PubMed] [Google Scholar]
- Milligan G., Tanfin Z., Goureau O., Unson C., Harbon S. Identification of both Gi2 and a novel, immunologically distinct, form of Go in rat myometrial membranes. FEBS Lett. 1989 Feb 27;244(2):411–416. doi: 10.1016/0014-5793(89)80574-5. [DOI] [PubMed] [Google Scholar]
- Mokhtari A., Do Khac L., Harbon S. Forskolin alters sensitivity of the cAMP-generating system to stimulatory as well as to inhibitory agonists. A study with intact human platelets and guinea pig myometrium. Eur J Biochem. 1988 Sep 1;176(1):131–137. doi: 10.1111/j.1432-1033.1988.tb14260.x. [DOI] [PubMed] [Google Scholar]
- Mokhtari A., Do Khac L., Tanfin Z., Harbon S. Forskolin modulates cyclic AMP generation in the rat myometrium. Interactions with isoproterenol and prostaglandins E2 and I2. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(3):213–227. [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Tanfin Z., Harbon S. Heterologous regulations of cAMP responses in pregnant rat myometrium. Evolution from a stimulatory to an inhibitory prostaglandin E2 and prostacyclin effect. Mol Pharmacol. 1987 Aug;32(1):249–257. [PubMed] [Google Scholar]
- Thomas A. P., Alexander J., Williamson J. R. Relationship between inositol polyphosphate production and the increase of cytosolic free Ca2+ induced by vasopressin in isolated hepatocytes. J Biol Chem. 1984 May 10;259(9):5574–5584. [PubMed] [Google Scholar]
- Van Sande J., Erneux C., Dumont J. E. Negative control of TSH action by iodide and acetylcholine: mechanism of action in intact thyroid cells. J Cyclic Nucleotide Res. 1977 Oct;3(5):335–345. [PubMed] [Google Scholar]
- Varol F. G., Hadjiconstantinou M., Zuspan F. P., Neff N. H. Pharmacological characterization of the muscarinic receptors mediating phosphoinositide hydrolysis in rat myometrium. J Pharmacol Exp Ther. 1989 Apr;249(1):11–15. [PubMed] [Google Scholar]
- Vesin M. F., Harbon S. The effects of epinephrine, prostaglandins, and their antagonists on adenosine cyclic 3',5'-monophosphate concentrations and motility of the rat uterus. Mol Pharmacol. 1974 May;10(3):457–473. [PubMed] [Google Scholar]
- Vesin M. F., Khac L. D., Harbon S. Prostacyclin as an endogenous modulator of adenosine cyclic 3',5'-monophosphate levels in rat myometrium and endometrium. Mol Pharmacol. 1979 Nov;16(3):823–840. [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]


