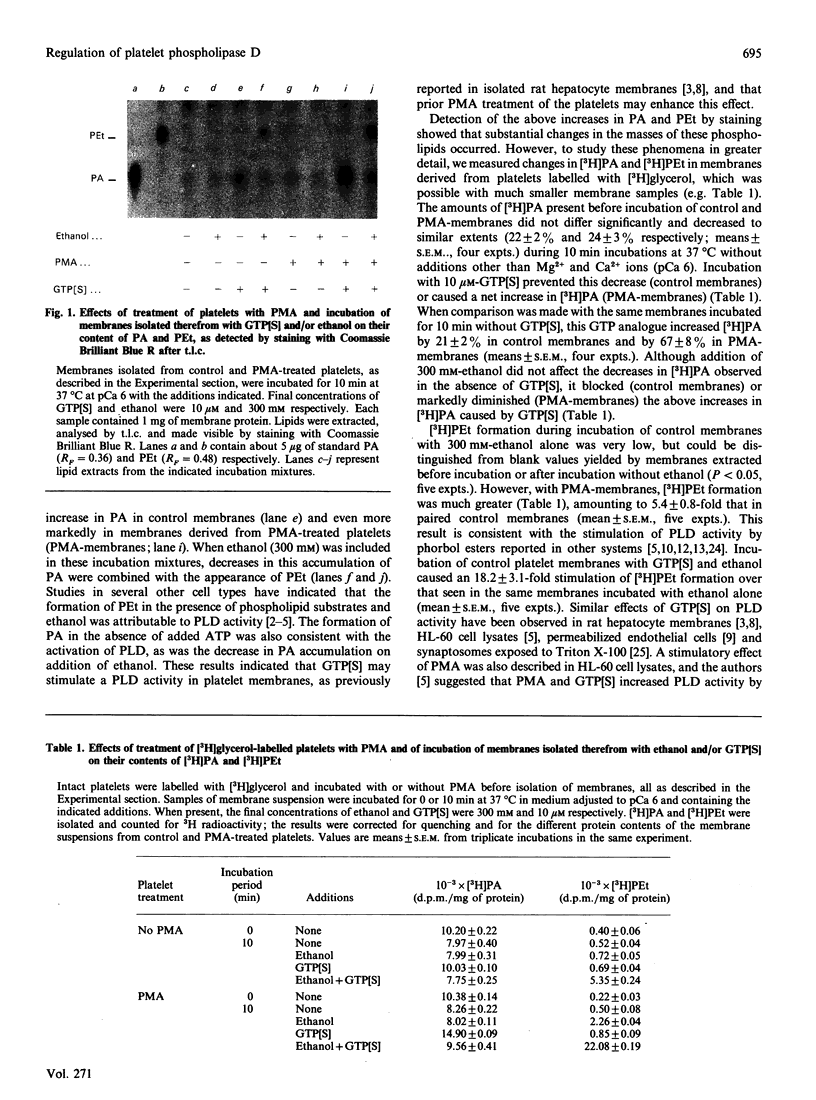
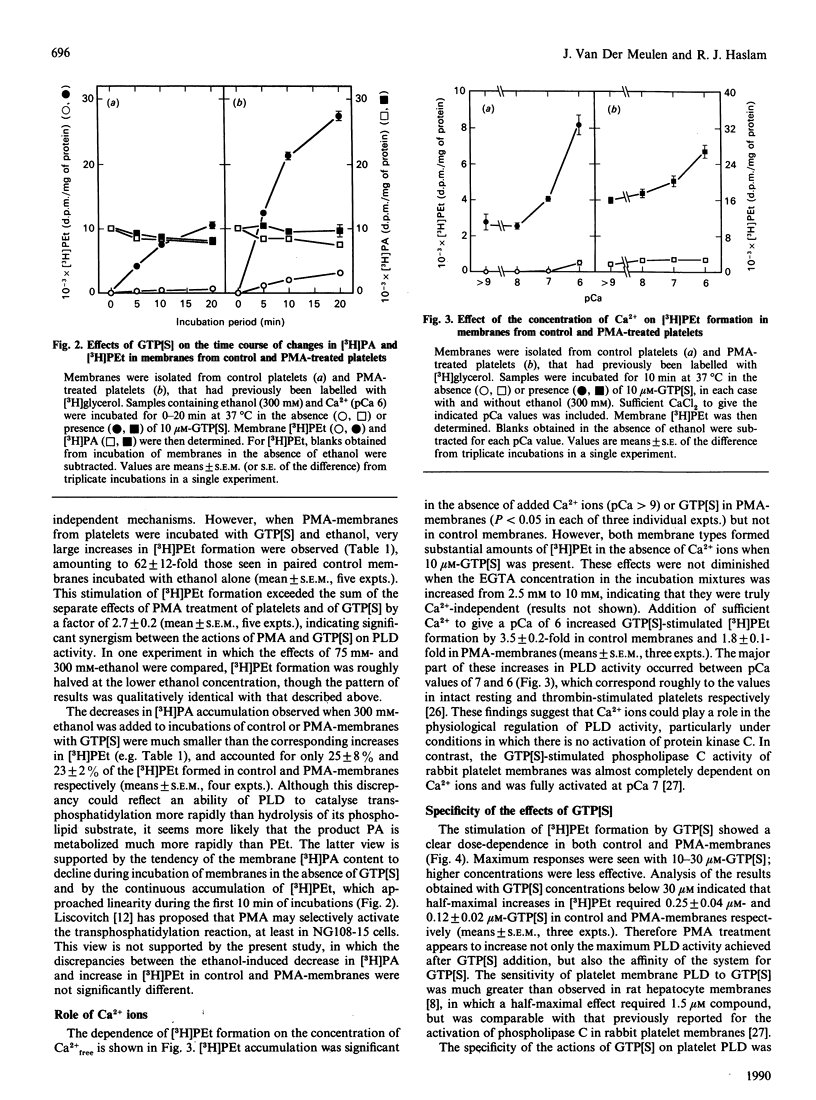
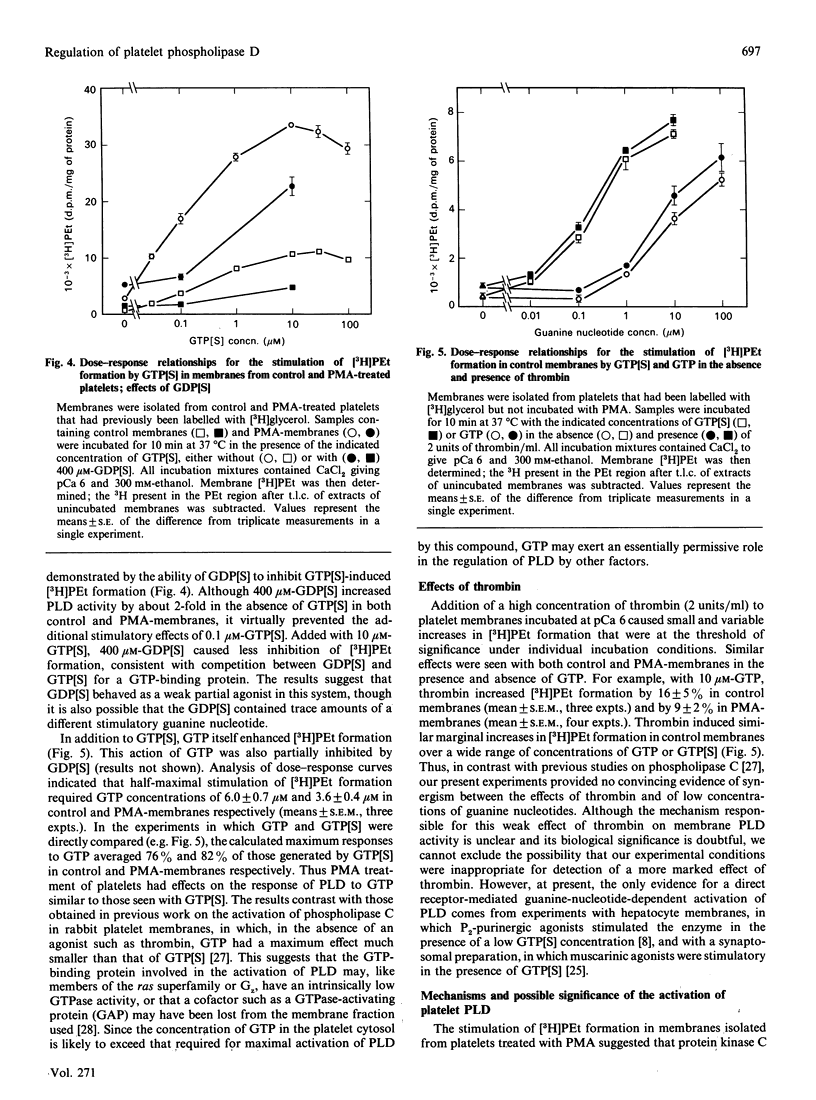
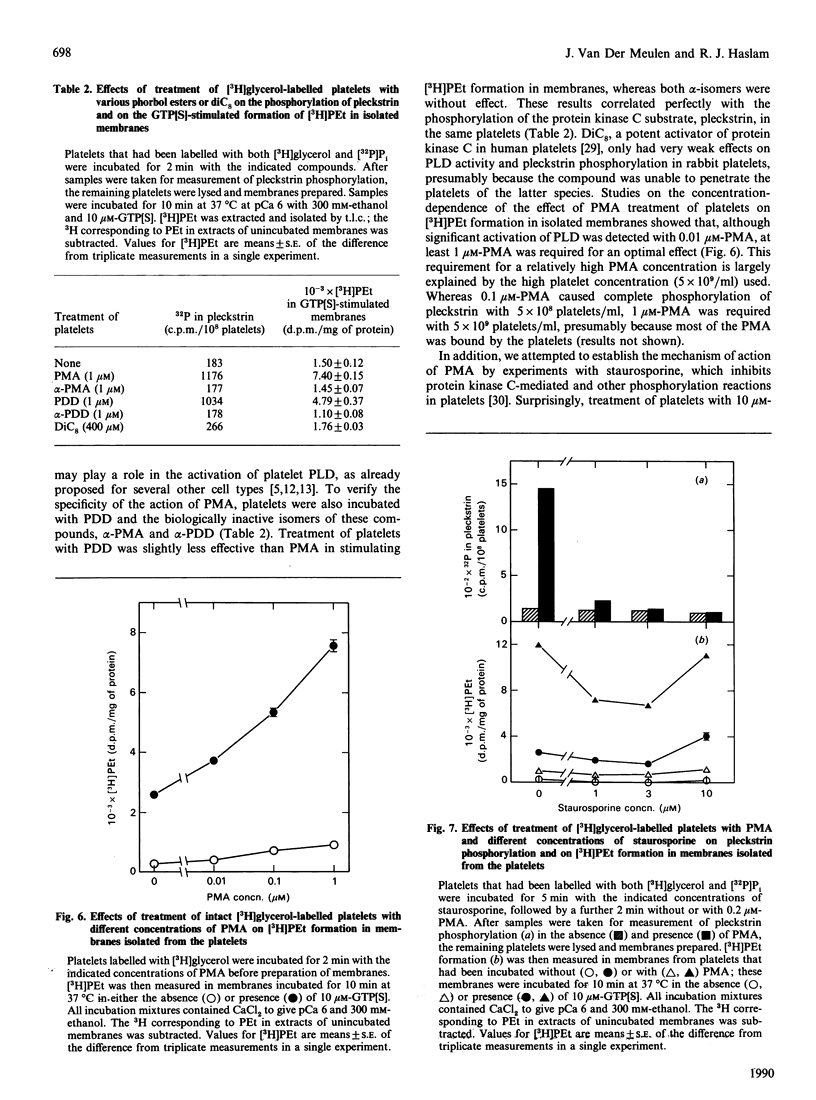
Abstract
Rabbit platelets were labelled with [3H]glycerol and incubated with or without phorbol 12-myristate 13-acetate (PMA). Membranes were then isolated and assayed for phospholipase D (PLD) activity by monitoring [3H]phosphatidylethanol formation in the presence of 300 mM-ethanol. At a [Ca2+free] of 1 microM, PLD activity was detected in control membranes, but was 5.4 +/- 0.8-fold (mean +/- S.E.M.) greater in membranes from PMA-treated platelets. Under the same conditions, 10 microM-guanosine 5'-[gamma-thio]triphosphate (GTP[S]) stimulated PLD by 18 +/- 3-fold in control membranes, whereas PMA treatment and GTP[S] interacted synergistically to increase PLD activity by 62 +/- 12-fold. GTP[S]-stimulated PLD activity was observed in the absence of Ca2+, but was increased by 1 microM-Ca2+ (3.5 +/- 0.2-fold and 1.8 +/- 0.1-fold in membranes from control and PMA-treated platelets respectively). GTP exerted effects almost as great as those of GTP[S], but 20-30-fold higher concentrations were required. Guanosine 5'-[beta-thio]diphosphate inhibited the effects of GTP[S] or GTP, suggesting a role for a GTP-binding protein in activation of PLD. Thrombin (2 units/ml) stimulated the PLD activity of platelet membranes only very weakly and in a GTP-independent manner. The actions of PMA and analogues on PLD activity correlated with their ability to stimulate protein kinase C in intact platelets. Staurosporine, a potent protein kinase inhibitor, had both inhibitory and, at higher concentrations, stimulatory effects on the activation of PLD by PMA. The results suggest that PMA not only stimulates PLD via activation of protein kinase C but can also activate the enzyme by a phosphorylation-independent mechanism in the presence of staurosporine. However, under physiological conditions, full activation of platelet PLD may require the interplay of protein kinase C, increased Ca2+ and a GTP-binding protein, and may occur as a secondary effect of the activation of phospholipase C.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTER R. H., JANDL J. H. PLATELET SEQUESTRATION IN MAN. I. METHODS. J Clin Invest. 1964 May;43:843–855. doi: 10.1172/JCI104970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardlie N. G., Packham M. A., Mustard J. F. Adenosine diphosphate-induced platelet aggregation in suspensions of washed rabbit platelets. Br J Haematol. 1970 Jul;19(1):7–17. doi: 10.1111/j.1365-2141.1970.tb01596.x. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baldassare J. J., Henderson P. A., Fisher G. J. Plasma membrane associated phospholipase C from human platelets: synergistic stimulation of phosphatidylinositol 4,5-bisphosphate hydrolysis by thrombin and guanosine 5'-O-(3-thiotriphosphate). Biochemistry. 1989 Jan 10;28(1):56–60. doi: 10.1021/bi00427a009. [DOI] [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Two roles for guanine nucleotides in the stimulus-secretion sequence of neutrophils. Nature. 1986 Feb 6;319(6053):504–507. doi: 10.1038/319504a0. [DOI] [PubMed] [Google Scholar]
- Ben-Av P., Liscovitch M. Phospholipase D activation by the mitogens platelet-derived growth factor and 12-O-tetradecanoylphorbol 13-acetate in NIH-3T3 cells. FEBS Lett. 1989 Dec 18;259(1):64–66. doi: 10.1016/0014-5793(89)81495-4. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Pai J. K., Mullmann T. J., Egan R. W., Siegel M. I. Regulation of phospholipase D in HL-60 granulocytes. Activation by phorbol esters, diglyceride, and calcium ionophore via protein kinase- independent mechanisms. J Biol Chem. 1989 May 25;264(15):9069–9076. [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Wilson P. B., Exton J. H. Phosphatidate accumulation in hormone-treated hepatocytes via a phospholipase D mechanism. J Biol Chem. 1987 Nov 5;262(31):15309–15315. [PubMed] [Google Scholar]
- Bocckino S. B., Wilson P. B., Exton J. H. Ca2+-mobilizing hormones elicit phosphatidylethanol accumulation via phospholipase D activation. FEBS Lett. 1987 Dec 10;225(1-2):201–204. doi: 10.1016/0014-5793(87)81157-2. [DOI] [PubMed] [Google Scholar]
- Cabot M. C., Welsh C. J., Zhang Z. C., Cao H. T. Evidence for a protein kinase C-directed mechanism in the phorbol diester-induced phospholipase D pathway of diacylglycerol generation from phosphatidylcholine. FEBS Lett. 1989 Mar 13;245(1-2):85–90. doi: 10.1016/0014-5793(89)80197-8. [DOI] [PubMed] [Google Scholar]
- Chalifour R., Kanfer J. N. Fatty acid activation and temperature perturbation of rat brain microsomal phospholipase D. J Neurochem. 1982 Aug;39(2):299–305. doi: 10.1111/j.1471-4159.1982.tb03946.x. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. Ca2+-dependent conversion of phosphatidylinositol to phosphatidate in neutrophils stimulated with fMet-Leu-Phe or ionophore A23187. Biochim Biophys Acta. 1984 Aug 15;795(1):37–46. doi: 10.1016/0005-2760(84)90102-4. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Howell T. W., Gomperts B. D. Two G-proteins act in series to control stimulus-secretion coupling in mast cells: use of neomycin to distinguish between G-proteins controlling polyphosphoinositide phosphodiesterase and exocytosis. J Cell Biol. 1987 Dec;105(6 Pt 1):2745–2750. doi: 10.1083/jcb.105.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Gruchalla R. S., Dinh T. T., Kennerly D. A. An indirect pathway of receptor-mediated 1,2-diacylglycerol formation in mast cells. I. IgE receptor-mediated activation of phospholipase D. J Immunol. 1990 Mar 15;144(6):2334–2342. [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M. Guanine nucleotides decrease the free [Ca2+] required for secretion of serotonin from permeabilized blood platelets. Evidence of a role for a GTP-binding protein in platelet activation. FEBS Lett. 1984 Aug 20;174(1):90–95. doi: 10.1016/0014-5793(84)81084-4. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hrbolich J. K., Culty M., Haslam R. J. Activation of phospholipase C associated with isolated rabbit platelet membranes by guanosine 5'-[gamma-thio]triphosphate and by thrombin in the presence of GTP. Biochem J. 1987 Apr 15;243(2):457–465. doi: 10.1042/bj2430457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaoka T., Lynham J. A., Haslam R. J. Purification and characterization of the 47,000-dalton protein phosphorylated during degranulation of human platelets. J Biol Chem. 1983 Sep 25;258(18):11404–11414. [PubMed] [Google Scholar]
- Kobayashi M., Kanfer J. N. Phosphatidylethanol formation via transphosphatidylation by rat brain synaptosomal phospholipase D. J Neurochem. 1987 May;48(5):1597–1603. doi: 10.1111/j.1471-4159.1987.tb05707.x. [DOI] [PubMed] [Google Scholar]
- Krishnamurthi S., Joseph S., Kakkar V. V. Synergistic potentiation of 5-hydroxytryptamine secretion by platelet agonists and phorbol myristate acetate despite inhibition of agonist-induced arachidonate/thromboxane and beta-thromboglobulin release and Ca2+ mobilization by phorbol myristate acetate. Biochem J. 1986 Aug 15;238(1):193–199. doi: 10.1042/bj2380193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthi S., Kakkar V. V. Phorbol ester treatment inhibits thrombin but not stable GTP analogue-induced platelet granule secretion despite inhibition of phosphatidate formation with both agonists. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1257–1264. doi: 10.1016/s0006-291x(88)80768-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lapetina E. G., Reep B., Ganong B. R., Bell R. M. Exogenous sn-1,2-diacylglycerols containing saturated fatty acids function as bioregulators of protein kinase C in human platelets. J Biol Chem. 1985 Feb 10;260(3):1358–1361. [PubMed] [Google Scholar]
- Liscovitch M. Phosphatidylethanol biosynthesis in ethanol-exposed NG108-15 neuroblastoma X glioma hybrid cells. Evidence for activation of a phospholipase D phosphatidyl transferase activity by protein kinase C. J Biol Chem. 1989 Jan 25;264(3):1450–1456. [PubMed] [Google Scholar]
- MOLNAR J., LORAND L. Studies on apyrases. Arch Biochem Biophys. 1961 May;93:353–363. doi: 10.1016/0003-9861(61)90278-8. [DOI] [PubMed] [Google Scholar]
- MacIntyre D. E., McNicol A., Drummond A. H. Tumour-promoting phorbol esters inhibit agonist-induced phosphatidate formation and Ca2+ flux in human platelets. FEBS Lett. 1985 Jan 28;180(2):160–164. doi: 10.1016/0014-5793(85)81063-2. [DOI] [PubMed] [Google Scholar]
- Mallows R. S., Bolton T. B. Relationship between stimulated phosphatidic acid production and inositol lipid hydrolysis in intestinal longitudinal smooth muscle from guinea pig. Biochem J. 1987 Jun 15;244(3):763–768. doi: 10.1042/bj2440763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. W., Michaelis K. P2-purinergic agonists stimulate phosphodiesteratic cleavage of phosphatidylcholine in endothelial cells. Evidence for activation of phospholipase D. J Biol Chem. 1989 May 25;264(15):8847–8856. [PubMed] [Google Scholar]
- Mauco G., Dangelmaier C. A., Smith J. B. Inositol lipids, phosphatidate and diacylglycerol share stearoylarachidonoylglycerol as a common backbone in thrombin-stimulated human platelets. Biochem J. 1984 Dec 15;224(3):933–940. doi: 10.1042/bj2240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Handa S. Coomassie brilliant blue staining of lipids on thin-layer plates. Anal Biochem. 1984 Nov 1;142(2):406–410. doi: 10.1016/0003-2697(84)90484-6. [DOI] [PubMed] [Google Scholar]
- Pai J. K., Siegel M. I., Egan R. W., Billah M. M. Phospholipase D catalyzes phospholipid metabolism in chemotactic peptide-stimulated HL-60 granulocytes. J Biol Chem. 1988 Sep 5;263(25):12472–12477. [PubMed] [Google Scholar]
- Qian Z., Drewes L. R. Muscarinic acetylcholine receptor regulates phosphatidylcholine phospholipase D in canine brain. J Biol Chem. 1989 Dec 25;264(36):21720–21724. [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Rittenhouse S. E., Sasson J. P. Mass changes in myoinositol trisphosphate in human platelets stimulated by thrombin. Inhibitory effects of phorbol ester. J Biol Chem. 1985 Jul 25;260(15):8657–8660. [PubMed] [Google Scholar]
- Rubin R. Phosphatidylethanol formation in human platelets: evidence for thrombin-induced activation of phospholipase D. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1090–1096. doi: 10.1016/s0006-291x(88)80744-7. [DOI] [PubMed] [Google Scholar]
- Santos E., Nebreda A. R. Structural and functional properties of ras proteins. FASEB J. 1989 Aug;3(10):2151–2163. doi: 10.1096/fasebj.3.10.2666231. [DOI] [PubMed] [Google Scholar]
- Stutchfield J., Cockcroft S. Guanine nucleotides stimulate polyphosphoinositide phosphodiesterase and exocytotic secretion from HL60 cells permeabilized with streptolysin O. Biochem J. 1988 Mar 1;250(2):375–382. doi: 10.1042/bj2500375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundler R., Papahadjopoulos D. Control of membrane fusion by phospholipid head groups. I. Phosphatidate/phosphatidylinositol specificity. Biochim Biophys Acta. 1981 Dec 21;649(3):743–750. doi: 10.1016/0005-2736(81)90179-6. [DOI] [PubMed] [Google Scholar]
- Tettenborn C. S., Mueller G. C. 12-O-tetradecanoylphorbol-13-acetate activates phosphatidylethanol and phosphatidylglycerol synthesis by phospholipase D in cell lysates. Biochem Biophys Res Commun. 1988 Aug 30;155(1):249–255. doi: 10.1016/s0006-291x(88)81076-3. [DOI] [PubMed] [Google Scholar]
- Tysnes O. B., Verhoeven A. J., Holmsen H. Rates of production and consumption of phosphatidic acid upon thrombin stimulation of human platelets. Eur J Biochem. 1988 May 16;174(1):75–79. doi: 10.1111/j.1432-1033.1988.tb14064.x. [DOI] [PubMed] [Google Scholar]
- Vallar L., Biden T. J., Wollheim C. B. Guanine nucleotides induce Ca2+-independent insulin secretion from permeabilized RINm5F cells. J Biol Chem. 1987 Apr 15;262(11):5049–5056. [PubMed] [Google Scholar]
- Watson S. P., McNally J., Shipman L. J., Godfrey P. P. The action of the protein kinase C inhibitor, staurosporine, on human platelets. Evidence against a regulatory role for protein kinase C in the formation of inositol trisphosphate by thrombin. Biochem J. 1988 Jan 15;249(2):345–350. doi: 10.1042/bj2490345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi J., Takai Y., Kaibuchi K., Sano K., Castagna M., Nishizuka Y. Synergistic functions of phorbol ester and calcium in serotonin release from human platelets. Biochem Biophys Res Commun. 1983 Apr 29;112(2):778–786. doi: 10.1016/0006-291x(83)91529-2. [DOI] [PubMed] [Google Scholar]



