Abstract
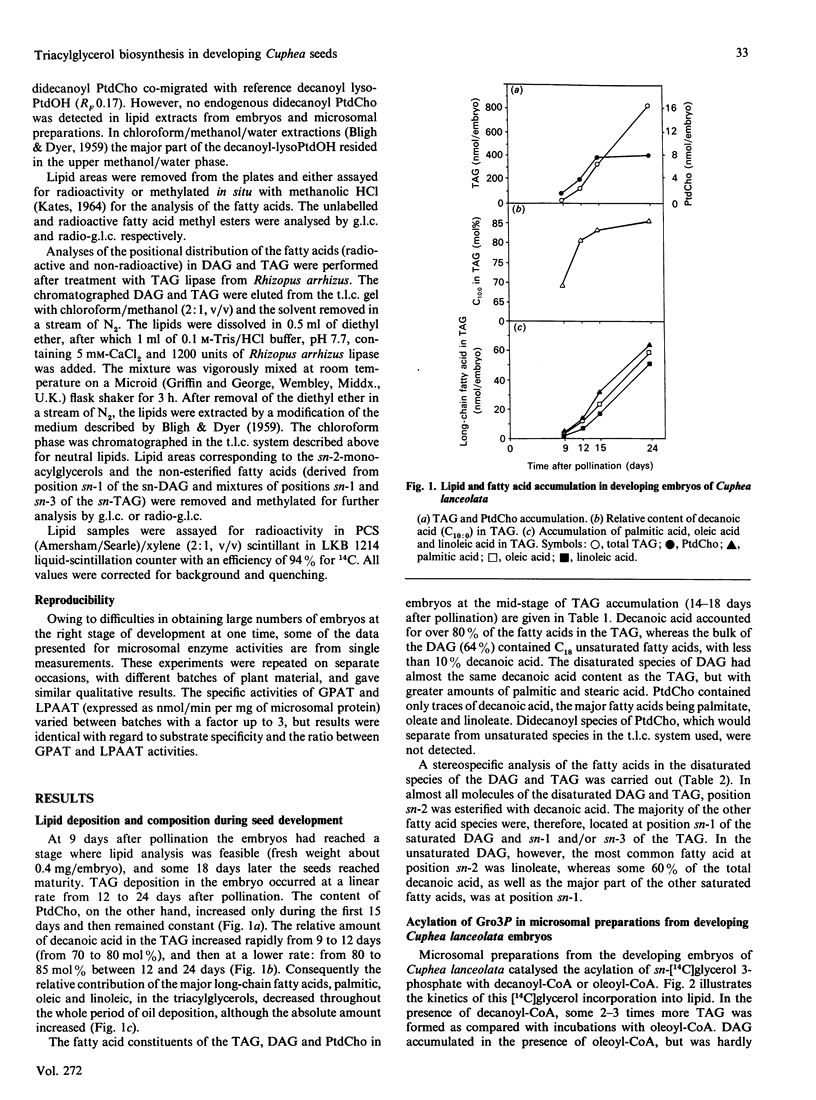
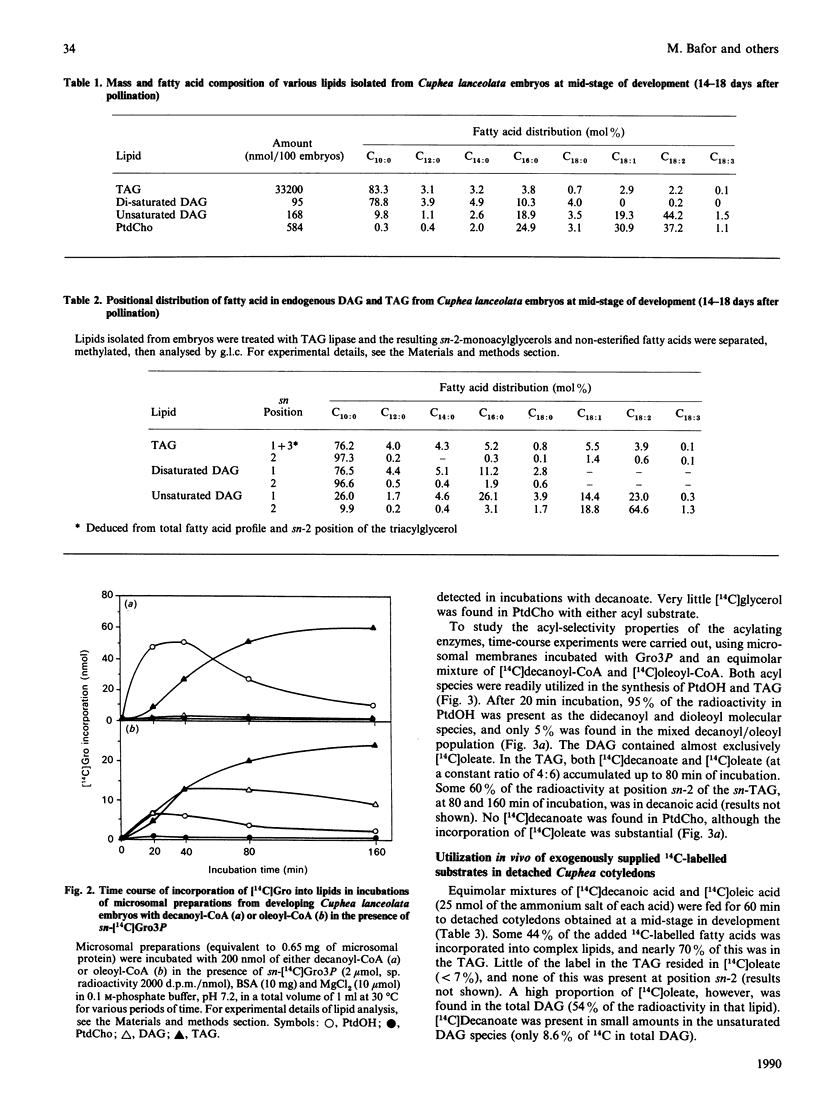
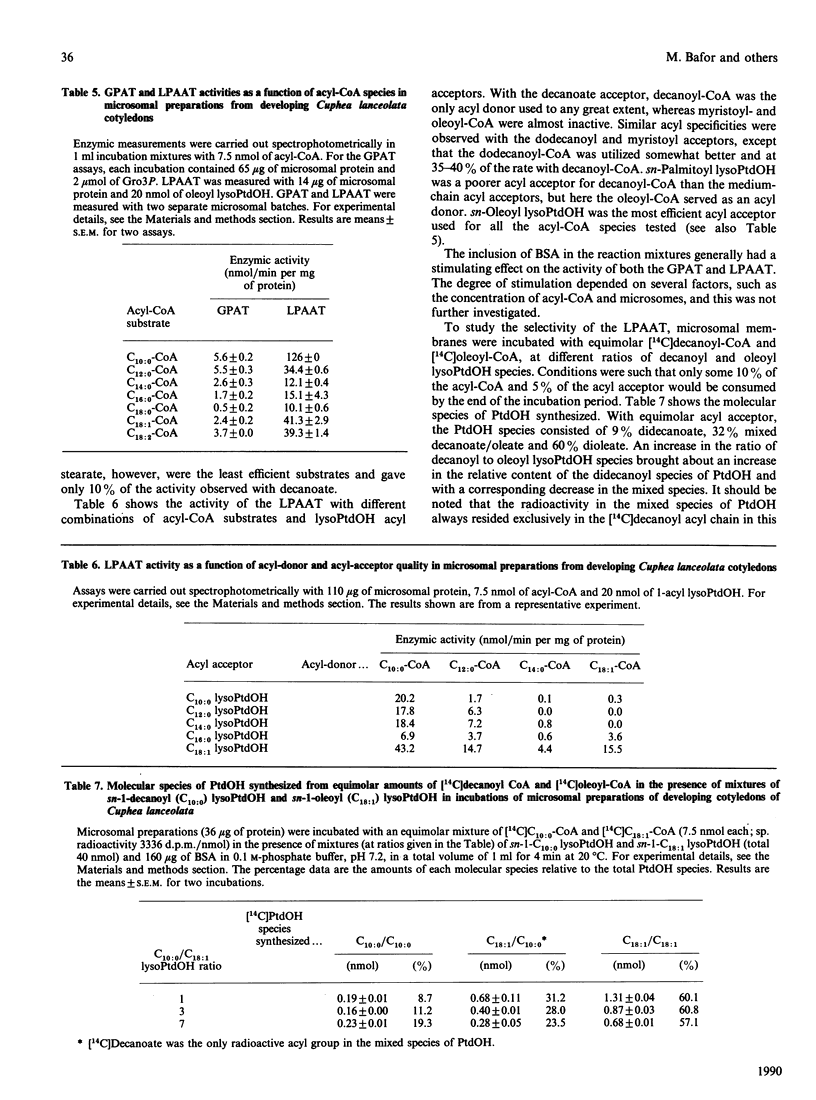
Embryos of Cuphea lanceolata have more than 80 mol% of decanoic acid ('capric acid') in their triacylglycerols, while this fatty acid is virtually absent in phosphatidylcholine (PtdCho). Seed development was complete 25-27 days after pollination, with rapid triacylglycerol deposition occurring between 9 and 24 days. PtdCho amounts increased until day 15 after pollination. Analysis of embryo lipids showed that the diacylglycerol (DAG) pool consisted of mainly long-chain molecular species, with a very small amount of mixed medium-chain/long-chain glycerols. Almost 100% of the fatty acid at position sn-2 in triacylglycerols (TAG) was decanoic acid. When equimolar mixtures of [14C]decanoic and [14C]oleic acid were fed to whole detached embryos, over half of the radioactivity in the DAG resided in [14C]oleate, whereas [14C]decanoic acid accounted for 93% of the label in the TAG. Microsomal preparations from developing embryos at the mid-stage of TAG accumulation catalysed the acylation of [14C]glycerol 3-phosphate with either decanoyl-CoA or oleoyl-CoA, resulting in the formation of phosphatidic acid (PtdOH), DAG and TAG. Very little [14C]glycerol entered PtdCho. In combined incubations, with an equimolar supply of [14C]oleoyl-CoA and [14C]decanoyl-CoA in the presence of glycerol 3-phosphate, the synthesized PtdCho species consisted to 95% of didecanoic and dioleic species. The didecanoyl-glycerols were very selectively utilized over the dioleoylglycerols in the production of TAG. Substantial amounts of [14C]oleate, but not [14C]decanoate, entered PtdCho. The microsomal preparations of developing embryos were used to assess the acyl specificities of the acyl-CoA:sn-glycerol-3-phosphate acyltransferase (GPAT, EC 2.3.1.15) and the acyl-CoA:sn-1-acyl-glycerol-3-phosphate acyltransferase (LPAAT, EC 2.3.1.51) in Cuphea lanceolata embryos. The efficiency of acyl-CoA utilization by the GPAT was in the order decanoyl = dodecanoyl greater than linoleoyl greater than myristoyl = oleoyl greater than palmitoyl. Decanoyl-CoA was the only acyl donor to be utilized to any extent by the LPAAT when sn-decanoylglycerol 3-phosphate was the acyl acceptor. sn-1-Acylglycerol 3-phosphates with acyl groups shorter than 16 carbon atoms did not serve as acyl acceptors for long-chain (greater than or equal to 16 carbon atoms) acyl-CoA species. On the basis of the results obtained, we propose a schematic model for triacylglycerol assembly and PtdCho synthesis in a tissue specialized in the synthesis of high amounts of medium-chain fatty acids.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cao Y. Z., Huang A. H. Acyl coenzyme a preference of diacylglycerol acyltransferase from the maturing seeds of cuphea, maize, rapeseed, and canola. Plant Physiol. 1987 Jul;84(3):762–765. doi: 10.1104/pp.84.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. P., Stumpf P. K. Fat metabolism in higher plants. XLIV. Fatty acid synthesis by a soluble fatty acid synthetase from Sclanum tuberosum. Arch Biochem Biophys. 1971 Apr;143(2):412–427. doi: 10.1016/0003-9861(71)90228-1. [DOI] [PubMed] [Google Scholar]
- Ichihara K., Asahi T., Fujii S. 1-Acyl-sn-glycerol-3-phosphate acyltransferase in maturing safflower seeds and its contribution to the non-random fatty acid distribution of triacylglycerol. Eur J Biochem. 1987 Sep 1;167(2):339–347. doi: 10.1111/j.1432-1033.1987.tb13342.x. [DOI] [PubMed] [Google Scholar]
- Ichihara K. sn-Glycerol-3-phosphate acyltransferase in a particulate fraction from maturing safflower seeds. Arch Biochem Biophys. 1984 Aug 1;232(2):685–698. doi: 10.1016/0003-9861(84)90589-7. [DOI] [PubMed] [Google Scholar]
- KATES M. SIMPLIFIED PROCEDURES FOR HYDROLYSIS OR METHANOLYSIS OF LIPIDS. J Lipid Res. 1964 Jan;5:132–135. [PubMed] [Google Scholar]
- KENNEDY E. P. Biosynthesis of complex lipids. Fed Proc. 1961 Dec;20:934–940. [PubMed] [Google Scholar]
- Kanda P., Wells M. A. Facile acylation of glycerophosphocholine catalyzed by trifluoroacetic anhydride. J Lipid Res. 1981 Jul;22(5):877–879. [PubMed] [Google Scholar]
- LANDS W. E., HART P. METABOLISM OF GLYCEROLIPIDS. VI. SPECIFICITIES OF ACYL COENZYME A: PHOSPHOLIPID ACYLTRANSFERASES. J Biol Chem. 1965 May;240:1905–1911. [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo K. C., Huang A. H. Lysophosphatidate acyltransferase activities in the microsomes from palm endosperm, maize scutellum, and rapeseed cotyledon of maturing seeds. Plant Physiol. 1989 Dec;91(4):1288–1295. doi: 10.1104/pp.91.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakata T., Stumpf P. K. The purification and function of acetyl coenzyme A:acyl carrier protein transacylase. J Biol Chem. 1983 Mar 25;258(6):3592–3598. [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Browse J. Evidence for an oleoyl phosphatidylcholine desaturase in microsomal preparations from cotyledons of safflower (Carthamus tinctorius) seed. Biochem J. 1979 Jun 1;179(3):649–656. doi: 10.1042/bj1790649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobart A. K., Stymne S. The interconversion of diacylglycerol and phosphatidylcholine during triacylglycerol production in microsomal preparations of developing cotyledons of safflower (Carthamus tinctorius L.). Biochem J. 1985 Nov 15;232(1):217–221. doi: 10.1042/bj2320217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne S., Appelqvist L. A. The biosynthesis of linoleate from oleoyl-CoA via oleoyl-phosphatidylcholine in microsomes of developing safflower seeds. Eur J Biochem. 1978 Oct;90(2):223–229. doi: 10.1111/j.1432-1033.1978.tb12594.x. [DOI] [PubMed] [Google Scholar]
- Sánchez M., Nicholls D. G., Brindley D. N. [The relationship between palmitoyl-coenzyme A synthetase activity and esterification of sn-glycerol 3-phosphate in rat liver mitochondria]. Biochem J. 1973 Apr;132(4):697–706. doi: 10.1042/bj1320697. [DOI] [PMC free article] [PubMed] [Google Scholar]


