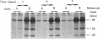Abstract
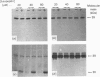
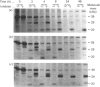
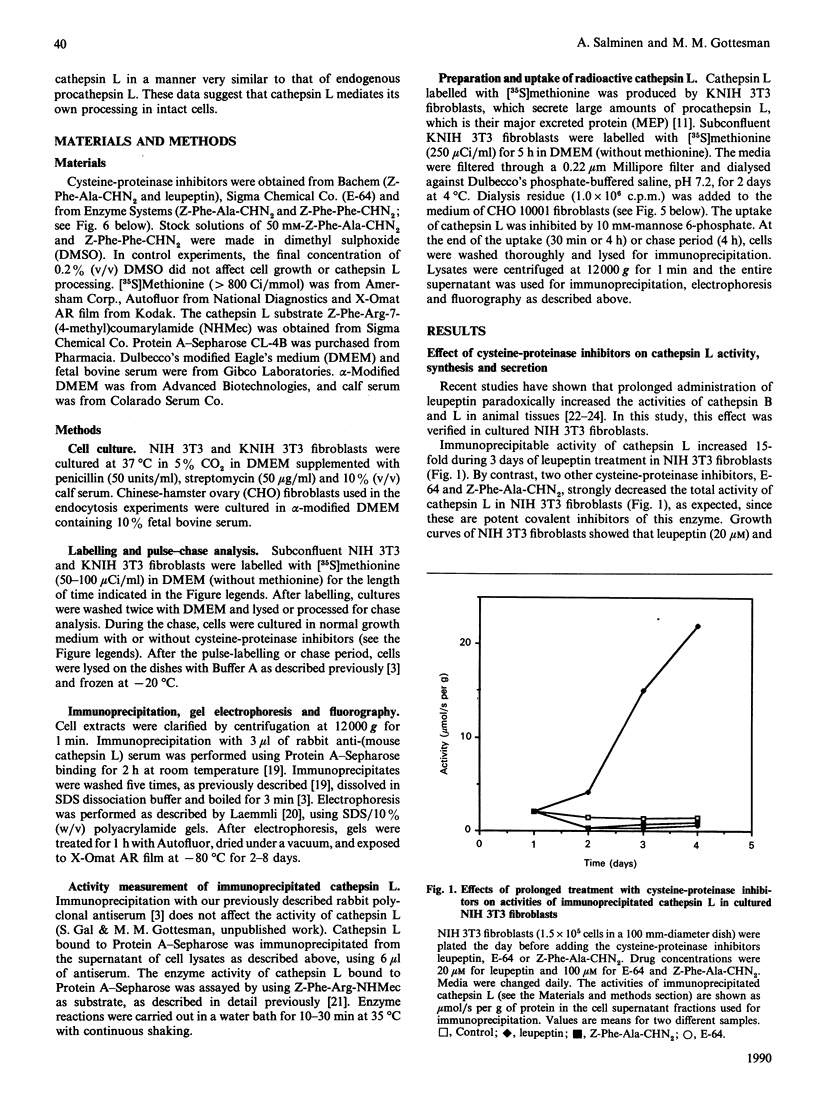
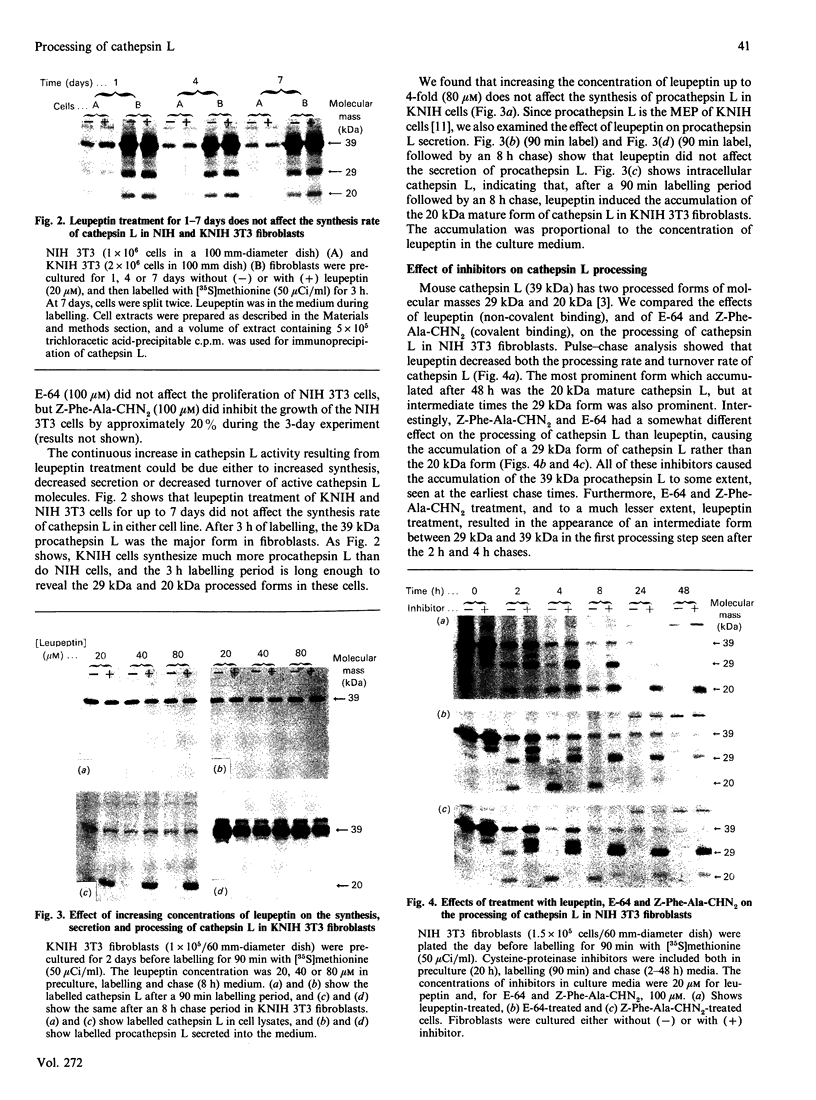
The lysosomal cysteine proteinase cathepsin L is synthesized in cultured mouse NIH 3T3 cells as a 39 kDa precursor and processed intracellularly into active 29 kDa and 20 kDa + 5 kDa lysosomal forms. Addition to culture media of the peptidyl aldehyde leupeptin, a non-covalent inhibitor of cathepsin L, results in the accumulation of the 20 kDa mature form of the enzyme, resulting in increased activity of cathepsin L as measured in an in vitro assay system in the absence of leupeptin. The more potent irreversible cathepsin L inhibitors benzyloxycarbonyl-Phe-Ala-diazomethane and L-transepoxysuccinyl-L-leucylamino-(4-guanidino)butane, when added to living cells at low concentrations, result in accumulation of all partially processed forms of cathepsin L, especially the 29 kDa form, suggesting that cathepsin L is responsible for its own processing. Exogenous procathepsin L introduced into CHO cells by endocytosis via the mannose 6-phosphate receptor is processed in a manner similar to endogenous procathepsin L. We conclude that the major intracellular pathway for processing of procathepsin L, either endogenous or exogenous, probably requires active cathepsin L.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Erickson A. H., Conner G. E., Blobel G. Biosynthesis of a lysosomal enzyme. Partial structure of two transient and functionally distinct NH2-terminal sequences in cathepsin D. J Biol Chem. 1981 Nov 10;256(21):11224–11231. [PubMed] [Google Scholar]
- Gal S., Gottesman M. M. The major excreted protein of transformed fibroblasts is an activable acid-protease. J Biol Chem. 1986 Feb 5;261(4):1760–1765. [PubMed] [Google Scholar]
- Gal S., Willingham M. C., Gottesman M. M. Processing and lysosomal localization of a glycoprotein whose secretion is transformation stimulated. J Cell Biol. 1985 Feb;100(2):535–544. doi: 10.1083/jcb.100.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieselmann V., Hasilik A., von Figura K. Processing of human cathepsin D in lysosomes in vitro. J Biol Chem. 1985 Mar 10;260(5):3215–3220. [PubMed] [Google Scholar]
- Gieselmann V., Pohlmann R., Hasilik A., Von Figura K. Biosynthesis and transport of cathepsin D in cultured human fibroblasts. J Cell Biol. 1983 Jul;97(1):1–5. doi: 10.1083/jcb.97.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M. Transformation-dependent secretion of a low molecular weight protein by murine fibroblasts. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2767–2771. doi: 10.1073/pnas.75.6.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. D., Shaw E. Peptidyl diazomethyl ketones are specific inactivators of thiol proteinases. J Biol Chem. 1981 Feb 25;256(4):1923–1928. [PubMed] [Google Scholar]
- Grinde B. The thiol proteinase inhibitors, Z-Phe-PheCHN2 and Z-Phe-AlaCHN2, inhibit lysosomal protein degradation in isolated rat hepatocytes. Biochim Biophys Acta. 1983 May 4;757(1):15–20. doi: 10.1016/0304-4165(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Langner J., Wiederanders B., Ansorge S., Bohley P. Cathepsin L. A new proteinase from rat-liver lysosomes. Eur J Biochem. 1977 Apr 1;74(2):293–301. doi: 10.1111/j.1432-1033.1977.tb11393.x. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Shaw E. Rapid interaction of cathepsin L by Z-Phe-PheCHN12 and Z-Phe-AlaCHN2. Biochem Biophys Res Commun. 1981 Jul 30;101(2):454–458. doi: 10.1016/0006-291x(81)91281-x. [DOI] [PubMed] [Google Scholar]
- Kominami E., Tsukahara T., Bando Y., Katunuma N. Autodegradation of lysosomal cysteine proteinases. Biochem Biophys Res Commun. 1987 Apr 29;144(2):749–756. doi: 10.1016/s0006-291x(87)80028-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemansky P., Gieselmann V., Hasilik A., von Figura K. Synthesis and transport of lysosomal acid phosphatase in normal and I-cell fibroblasts. J Biol Chem. 1985 Jul 25;260(15):9023–9030. [PubMed] [Google Scholar]
- Mason R. W., Wilcox D., Wikstrom P., Shaw E. N. The identification of active forms of cysteine proteinases in Kirsten-virus-transformed mouse fibroblasts by use of a specific radiolabelled inhibitor. Biochem J. 1989 Jan 1;257(1):125–129. doi: 10.1042/bj2570125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Kawabata T., Furuno K., Kato K. Evidence that aspartic proteinase is involved in the proteolytic processing event of procathepsin L in lysosomes. Arch Biochem Biophys. 1989 Jun;271(2):400–406. doi: 10.1016/0003-9861(89)90289-0. [DOI] [PubMed] [Google Scholar]
- Noda T., Isogai K., Katunuma N., Tarumoto Y., Ohzeki M. Effects of cathepsin B, H, and D in pectoral muscle of dystrophic chickens (line 413) of in vivo administration of E-64-c (N-[N-(L-3-transcarboxyoxirane-2-carbonyl)-L-leucyl]-3-methyl-butylamine). J Biochem. 1981 Sep;90(3):893–896. doi: 10.1093/oxfordjournals.jbchem.a133548. [DOI] [PubMed] [Google Scholar]
- Oude Elferink R. P., Van Doorn-Van Wakeren J., Hendriks T., Strijland A., Tager J. M. Transport and processing of endocytosed lysosomal alpha-glucosidase in cultured human skin fibroblasts. Eur J Biochem. 1986 Jul 15;158(2):339–344. doi: 10.1111/j.1432-1033.1986.tb09756.x. [DOI] [PubMed] [Google Scholar]
- Richardson J. M., Woychik N. A., Ebert D. L., Dimond R. L., Cardelli J. A. Inhibition of early but not late proteolytic processing events leads to the missorting and oversecretion of precursor forms of lysosomal enzymes in Dictyostelium discoideum. J Cell Biol. 1988 Dec;107(6 Pt 1):2097–2107. doi: 10.1083/jcb.107.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. G., Kreibich G., Popov D., Kato K., Sabatini D. D. Biosynthesis of lysosomal hydrolases: their synthesis in bound polysomes and the role of co- and post-translational processing in determining their subcellular distribution. J Cell Biol. 1982 Apr;93(1):135–143. doi: 10.1083/jcb.93.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A. Effects of the protease inhibitor leupeptin on proteolytic activities and regeneration of mouse skeletal muscles after exercise injuries. Am J Pathol. 1984 Oct;117(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- Shaw E., Dean R. T. The inhibition of macrophage protein turnover by a selective inhibitor of thiol proteinases. Biochem J. 1980 Feb 15;186(2):385–390. doi: 10.1042/bj1860385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Gottesman M. M. Activity and deletion analysis of recombinant human cathepsin L expressed in Escherichia coli. J Biol Chem. 1989 Dec 5;264(34):20487–20495. [PubMed] [Google Scholar]
- Smith S. M., Kane S. E., Gal S., Mason R. W., Gottesman M. M. Glycosylation of procathepsin L does not account for species molecular-mass differences and is not required for proteolytic activity. Biochem J. 1989 Sep 15;262(3):931–938. doi: 10.1042/bj2620931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J. H., Greenbaum L. M. Paradoxical effect of leupeptin in vivo on cathepsin B activity. Biochem Biophys Res Commun. 1983 Jan 14;110(1):332–338. doi: 10.1016/0006-291x(83)91300-1. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ikegaki N., Ichihara A. Purification and characterization of hemoglobin-hydrolyzing acidic thiol protease induced by leupeptin in rat liver. J Biol Chem. 1984 May 10;259(9):5937–5944. [PubMed] [Google Scholar]
- Troen B. R., Ascherman D., Atlas D., Gottesman M. M. Cloning and expression of the gene for the major excreted protein of transformed mouse fibroblasts. A secreted lysosomal protease regulated by transformation. J Biol Chem. 1988 Jan 5;263(1):254–261. [PubMed] [Google Scholar]
- Ward W. F., Chua B. L., Li J. B., Morgan H. E., Mortimore G. E. Inhibition of basal and deprivation-induced proteolysis by leupeptin and pepstatin in perfused rat liver and heart. Biochem Biophys Res Commun. 1979 Mar 15;87(1):92–98. doi: 10.1016/0006-291x(79)91651-6. [DOI] [PubMed] [Google Scholar]