Abstract
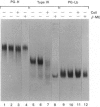
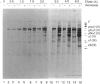
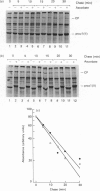
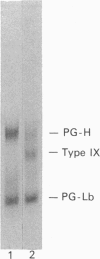
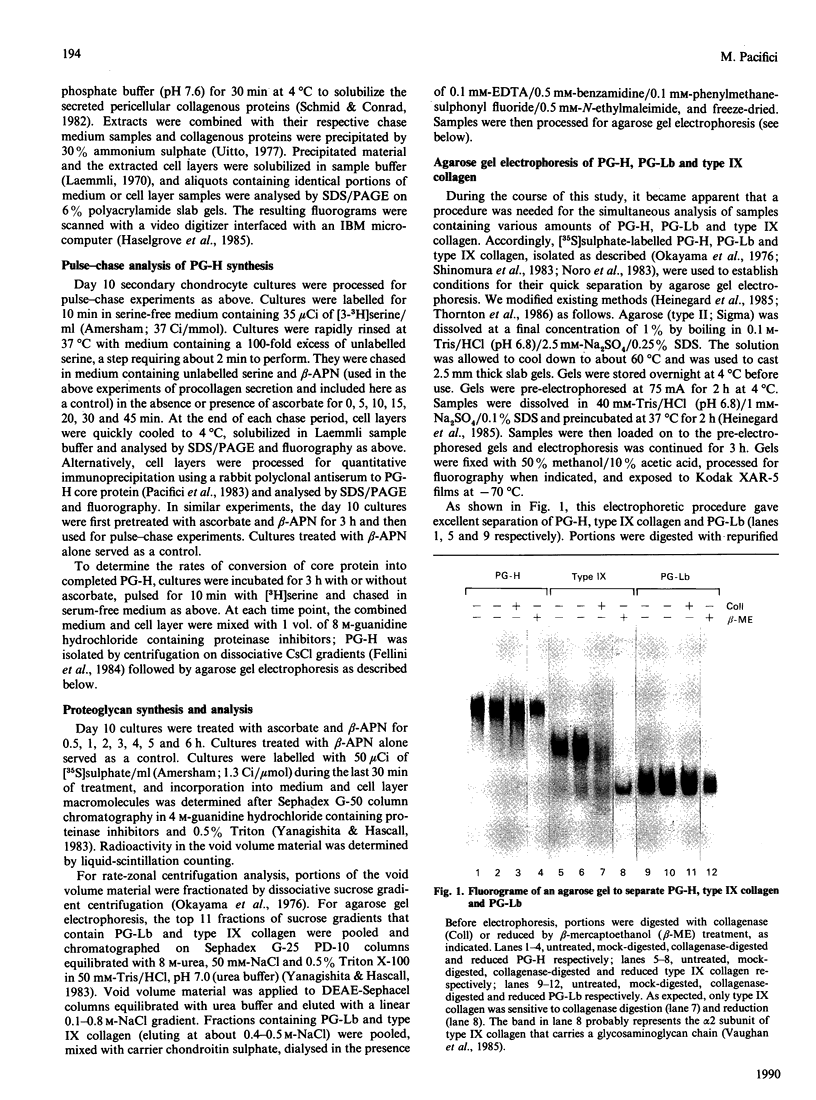
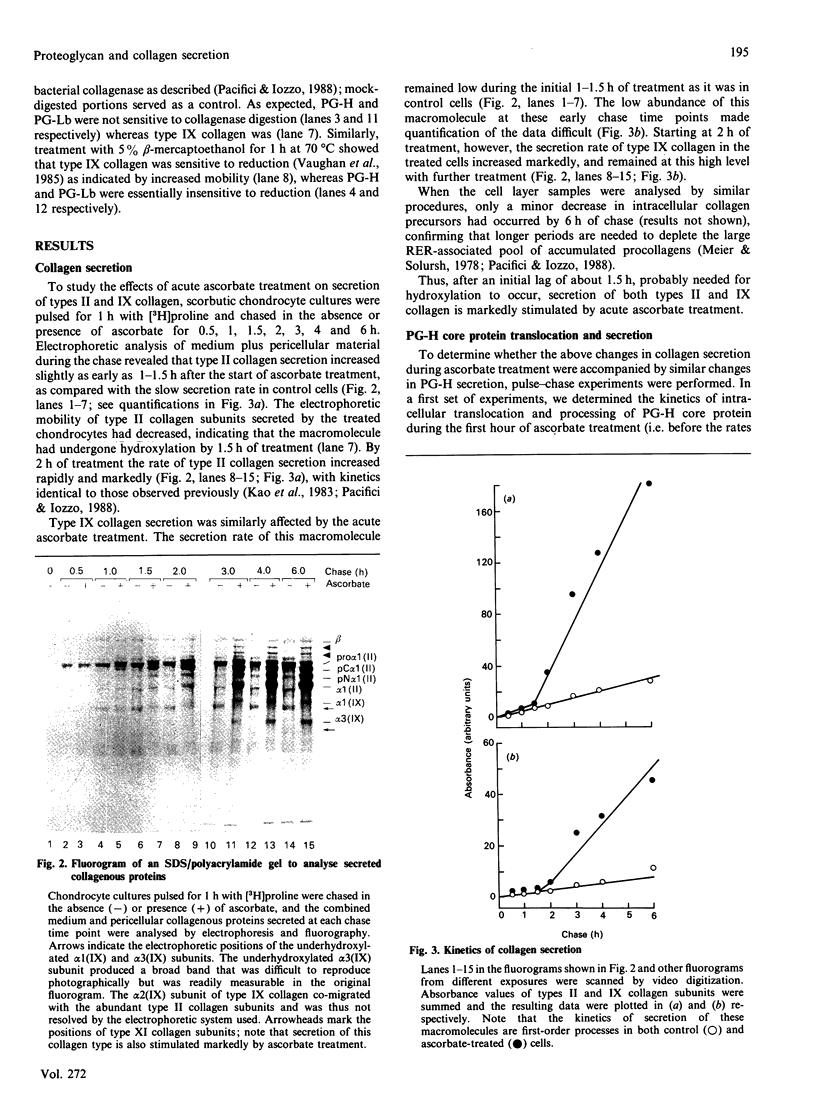
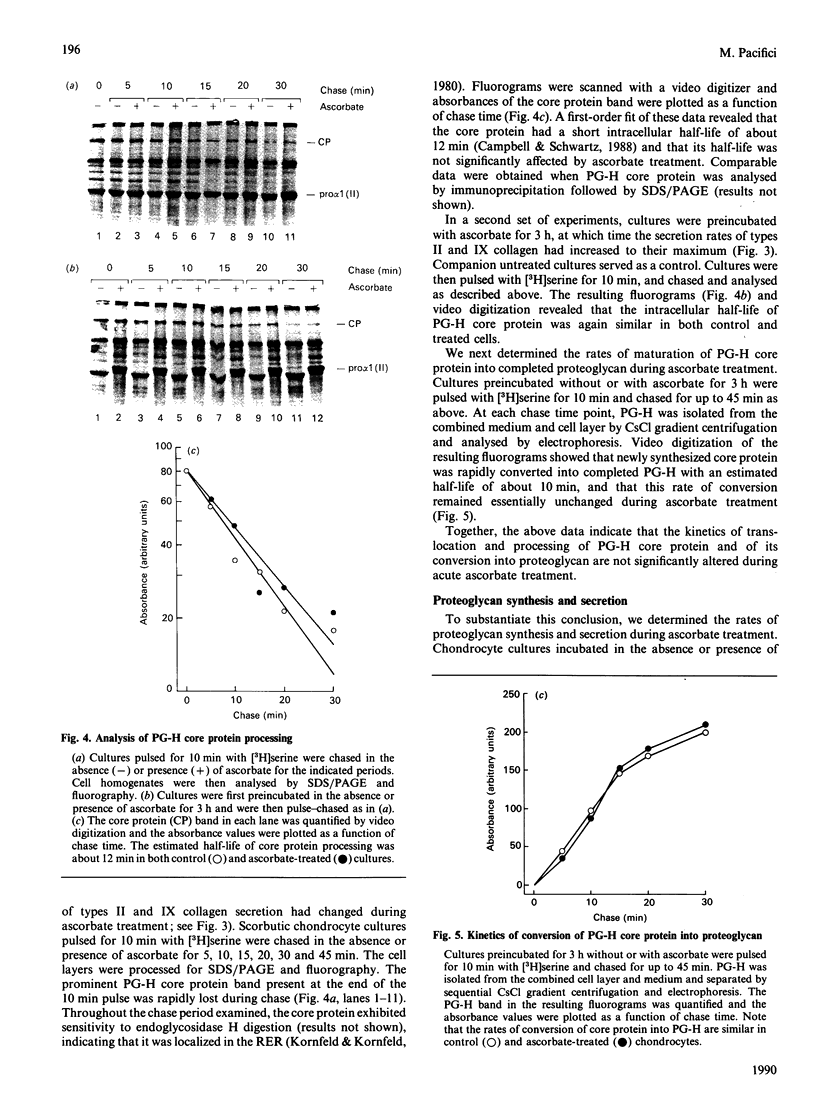
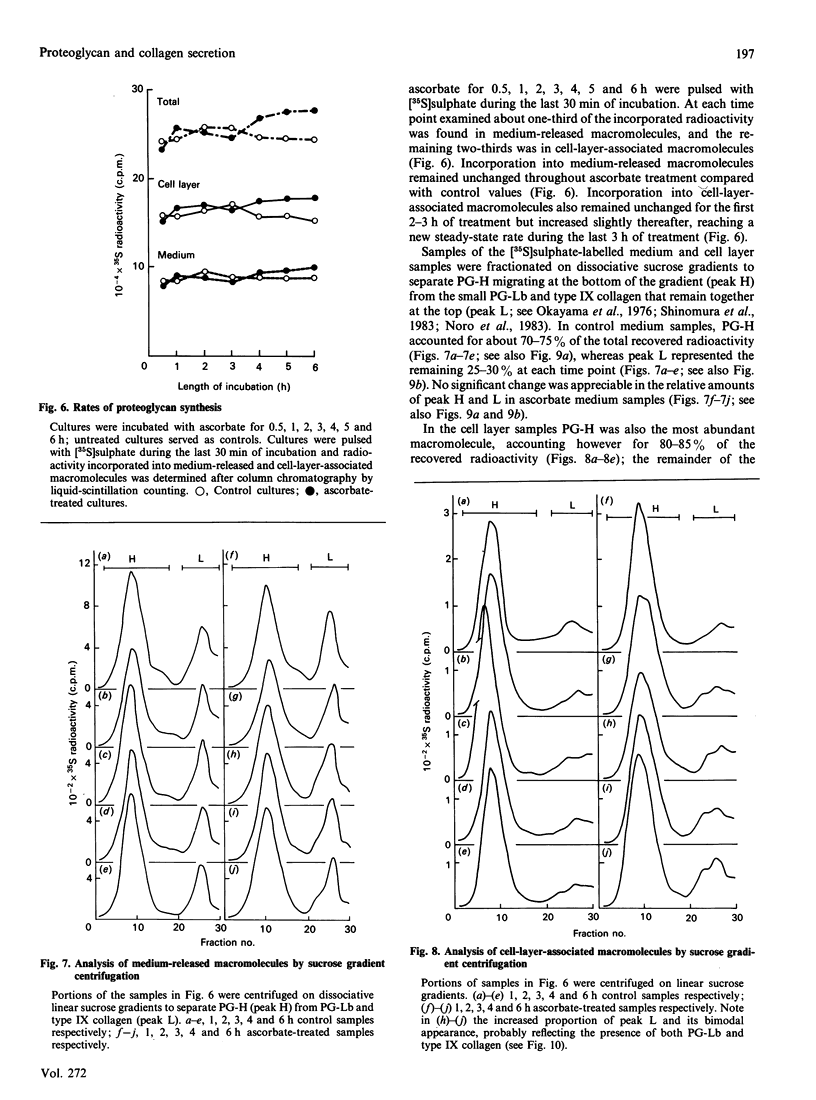
The mechanisms regulating the secretion of proteoglycans and collagens in chondrocytes, in particular those operating at the level of the rough endoplasmic reticulum (RER), are largely unknown. To examine these mechanisms, I studied the effects of acute ascorbate treatment on the secretion of two collagen types (types II and IX) and two proteoglycan types (PG-H and PG-Lb, the major keratan sulphate/chondroitin sulphate proteoglycan and the minor chondroitin sulphate proteoglycan respectively in cartilage) in scorbutic cultures of chick vertebral chondrocytes. I found that the scorbutic chondrocytes synthesized underhydroxylated precursors of types II and IX collagen that were secreted very slowly and accumulated in the RER. When the cultures were treated acutely with ascorbate, both macromolecules underwent hydroxylation within 1-1.5 h of treatment, and began to be secreted at normal high rates starting at about 2 h. Proteoglycan synthesis and secretion, however, remained largely unaffected by ascorbate treatment. Both the half-time of newly synthesized PG-H core protein in the RER and its conversion into completed proteoglycan were unchanged during treatment. Similarly, the overall rates of synthesis and secretion of both PG-H and PG-Lb remained at control levels during treatment. The data indicate that secretion of types II and IX collagen is regulated independently of secretion of PG-H and PG-Lb. This may be mediated by the ability of the RER of the chondrocyte to discriminate between procollagens and proteoglycan core proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. A., Prockop D. J. Affinity column purification of protocollagen proline hydroxylase from chick embryos and further characterization of the enzyme. J Biol Chem. 1973 Feb 25;248(4):1175–1182. [PubMed] [Google Scholar]
- Campbell S. C., Schwartz N. B. Kinetics of intracellular processing of chondroitin sulfate proteoglycan core protein and other matrix components. J Cell Biol. 1988 Jun;106(6):2191–2202. doi: 10.1083/jcb.106.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutroneo K. R., Guzman N. A., Sharawy M. M. Evidence for a subcellular vesicular site of collagen prolyl hydroxylation. J Biol Chem. 1974 Sep 25;249(18):5989–5994. [PubMed] [Google Scholar]
- Fellini S. A., Hascall V. C., Kimura J. H. Localization of proteoglycan core protein in subcellular fractions isolated from rat chondrosarcoma chondrocytes. J Biol Chem. 1984 Apr 10;259(7):4634–4641. [PubMed] [Google Scholar]
- Fitting T., Kabat D. Evidence for a glycoprotein "signal" involved in transport between subcellular organelles. Two membrane glycoproteins encoded by murine leukemia virus reach the cell surface at different rates. J Biol Chem. 1982 Dec 10;257(23):14011–14017. [PubMed] [Google Scholar]
- Fries E., Gustafsson L., Peterson P. A. Four secretory proteins synthesized by hepatocytes are transported from endoplasmic reticulum to Golgi complex at different rates. EMBO J. 1984 Jan;3(1):147–152. doi: 10.1002/j.1460-2075.1984.tb01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C., Lyons G., Rubenstein N., Kelly A. A rapid, inexpensive, quantitative, general-purpose densitometer and its application to one-dimensional gel electrophoretograms. Anal Biochem. 1985 Nov 1;150(2):449–456. doi: 10.1016/0003-2697(85)90534-2. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Sommarin Y., Hedbom E., Wieslander J., Larsson B. Assay of proteoglycan populations using agarose-polyacrylamide gel electrophoresis. Anal Biochem. 1985 Nov 15;151(1):41–48. doi: 10.1016/0003-2697(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V., Pacifici M. Ultrastructural localization of the major proteoglycan and type II procollagen in organelles and extracellular matrix of cultured chondroblasts. Histochemistry. 1986;86(2):113–122. doi: 10.1007/BF00493375. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., Harsch M., Murphy L., Rosenbloom J. Effects of temperature on conformation, hydroxylation, and secretion of chick tendon procollagen. J Biol Chem. 1974 Jul 25;249(14):4480–4486. [PubMed] [Google Scholar]
- Jimenez S. A., Yankowski R. Role of molecular conformation on secretion of chick tendon procollagen. J Biol Chem. 1978 Mar 10;253(5):1420–1426. [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Formation of enzyme-substrate complexes with protocollagen proline hydroxylase and large polypeptide substrates. J Biol Chem. 1969 Dec 10;244(23):6486–6492. [PubMed] [Google Scholar]
- Kao W. W., Mai S. H., Chou K. L., Ebert J. Mechanism for the regulation of post-translational modifications of procollagens synthesized by matrix-free cells from chick embryos. J Biol Chem. 1983 Jun 25;258(12):7779–7787. [PubMed] [Google Scholar]
- Kim J. J., Conrad H. E. Proteochondroitin sulfate synthesis in subcultured chick embryo tibial chondrocytes. J Biol Chem. 1982 Feb 25;257(4):1670–1675. [PubMed] [Google Scholar]
- Kim J. J., Conrad H. E. Secretion of chondroitin SO4 by monolayer cultures of chick embryo chondrocytes. J Biol Chem. 1980 Feb 25;255(4):1586–1597. [PubMed] [Google Scholar]
- Kreis T. E., Lodish H. F. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986 Sep 12;46(6):929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leboy P. S., Vaias L., Uschmann B., Golub E., Adams S. L., Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J Biol Chem. 1989 Oct 15;264(29):17281–17286. [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Snider M., Strous G. J. Hepatoma secretory proteins migrate from rough endoplasmic reticulum to Golgi at characteristic rates. Nature. 1983 Jul 7;304(5921):80–83. doi: 10.1038/304080a0. [DOI] [PubMed] [Google Scholar]
- Meier S., Solursh M. Ultrastructural analysis of the effect of ascorbic acid on secretion and assembly of extracellular matrix by cultured chick embryo chondrocytes. J Ultrastruct Res. 1978 Oct;65(1):48–59. doi: 10.1016/s0022-5320(78)90021-7. [DOI] [PubMed] [Google Scholar]
- Mitchell D., Hardingham T. The effects of cycloheximide on the biosynthesis and secretion of proteoglycans by chondrocytes in culture. Biochem J. 1981 May 15;196(2):521–529. doi: 10.1042/bj1960521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noro A., Kimata K., Oike Y., Shinomura T., Maeda N., Yano S., Takahashi N., Suzuki S. Isolation and characterization of a third proteoglycan (PG-Lt) from chick embryo cartilage which contains disulfide-bonded collagenous polypeptide. J Biol Chem. 1983 Aug 10;258(15):9323–9331. [PubMed] [Google Scholar]
- Okayama M., Pacifici M., Holtzer H. Differences among sulfated proteoglycans synthesized in nonchondrogenic cells, presumptive chondroblasts, and chondroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3224–3228. doi: 10.1073/pnas.73.9.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R., Berg R. A., Kishida Y., Prockop D. J. Further characterization of embryonic tendon fibroblasts and the use of immunoferritin techniques to study collagen biosynthesis. J Cell Biol. 1975 Feb;64(2):340–355. doi: 10.1083/jcb.64.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERKOFSKY B., UDENFRIEND S. ENZYMATIC HYDROXYLATION OF PROLINE IN MICROSOMAL POLYPEPTIDE LEADING TO FORMATION OF COLLAGEN. Proc Natl Acad Sci U S A. 1965 Feb;53:335–342. doi: 10.1073/pnas.53.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici M., Iozzo R. V. Remodeling of the rough endoplasmic reticulum during stimulation of procollagen secretion by ascorbic acid in cultured chondrocytes. A biochemical and morphological study. J Biol Chem. 1988 Feb 15;263(5):2483–2492. [PubMed] [Google Scholar]
- Pacifici M., Soltesz R., Thal G., Shanley D. J., Boettiger D., Holtzer H. Immunological characterization of the major chick cartilage proteoglycan and its intracellular localization in cultured chondroblasts: a comparison with Type II procollagen. J Cell Biol. 1983 Dec;97(6):1724–1736. doi: 10.1083/jcb.97.6.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajaniemi T., Helaakoski T., Tasanen K., Myllylä R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987 Mar;6(3):643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese C. A., Mayne R. Minor collagens of chicken hyaline cartilage. Biochemistry. 1981 Sep 15;20(19):5443–5448. doi: 10.1021/bi00522a014. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J., Harsch M., Jimenez S. Hydroxyproline content determines the denaturation temperature of chick tendon collagen. Arch Biochem Biophys. 1973 Oct;158(2):478–484. doi: 10.1016/0003-9861(73)90539-0. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Schmid T. M., Conrad H. E. A unique low molecular weight collagen secreted by cultured chick embryo chondrocytes. J Biol Chem. 1982 Oct 25;257(20):12444–12450. [PubMed] [Google Scholar]
- Shinomura T., Kimata K., Oike Y., Noro A., Hirose N., Tanabe K., Suzuki S. The occurrence of three different proteoglycan species in chick embryo cartilage. Isolation and characterization of a second proteoglycan (PG-Lb) and its precursor form. J Biol Chem. 1983 Aug 10;258(15):9314–9322. [PubMed] [Google Scholar]
- Sifers R. N., Brashears-Macatee S., Kidd V. J., Muensch H., Woo S. L. A frameshift mutation results in a truncated alpha 1-antitrypsin that is retained within the rough endoplasmic reticulum. J Biol Chem. 1988 May 25;263(15):7330–7335. [PubMed] [Google Scholar]
- Thornton D. J., Nieduszynski I. A., Oates K., Sheehan J. K. Electron-microscopic and electrophoretic studies of bovine femoral-head cartilage proteoglycan fractions. Biochem J. 1986 Nov 15;240(1):41–48. doi: 10.1042/bj2400041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J. Biosynthesis of type II collagen. Removal of amino-and carboxy-terminal extensions from procollagen synthesized by chick embryo cartilage cells. Biochemistry. 1977 Jul 26;16(15):3421–3429. doi: 10.1021/bi00634a020. [DOI] [PubMed] [Google Scholar]
- Vaughan L., Winterhalter K. H., Bruckner P. Proteoglycan Lt from chicken embryo sternum identified as type IX collagen. J Biol Chem. 1985 Apr 25;260(8):4758–4763. [PubMed] [Google Scholar]
- Vertel B. M., Morrell J. J., Barkman L. L. Immunofluorescence studies on cartilage matrix synthesis. The synthesis of link protein, chondroitin sulfate proteoglycan monomer and type II collagen. Exp Cell Res. 1985 Jun;158(2):423–432. doi: 10.1016/0014-4827(85)90466-5. [DOI] [PubMed] [Google Scholar]
- Vertel B. M., Velasco A., LaFrance S., Walters L., Kaczman-Daniel K. Precursors of chondroitin sulfate proteoglycan are segregated within a subcompartment of the chondrocyte endoplasmic reticulum. J Cell Biol. 1989 Oct;109(4 Pt 1):1827–1836. doi: 10.1083/jcb.109.4.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Characterization of low buoyant density dermatan sulfate proteoglycans synthesized by rat ovarian granulosa cells in culture. J Biol Chem. 1983 Nov 10;258(21):12847–12856. [PubMed] [Google Scholar]