Abstract
1. Portacaval shunting in rats results in several metabolic alterations similar to those seen in patients with hepatic encephalopathy. The characteristic changes include: (a) diminution of cerebral function; (b) raised plasma ammonia and brain glutamine levels; (c) increased neutral amino acid transport across the blood-brain barrier; (d) altered brain and plasma amino acid levels; and (e) changes in brain neurotransmitter content. The aetiology of these abnormalities remains unknown. 2. To study the degree to which ammonia could be responsible, rats were made hyperammonaemic by administering 40 units of urease/kg body weight every 12 h and killing the rats 48 h after the first injection. 3. The changes observed in the urease-treated rats were: (a) whole-brain glucose use was significantly depressed, whereas the levels of high-energy phosphates remained unchanged; (b) the permeability of the blood-brain to barrier to two large neutral amino acids, tryptophan and leucine, was increased; (c) blood-brain barrier integrity was maintained, as indicated by the unchanged permeability-to-surface-area product for acetate; (d) plasma and brain amino acid concentrations were altered; and (e) dopamine, 5-hydroxytryptamine (serotonin) and noradrenaline levels in brain were unchanged, but 5-hydroxyindoleacetic acid (5-HIAA), a metabolite of 5-hydroxytryptamine, was elevated. 4. The depressed brain glucose use, increased tryptophan permeability-to-surface-area product, elevated brain tryptophan content and rise in the level of cerebral 5-HIAA were closely correlated with the observed rise in brain glutamine content. 5. These results suggest that many of the metabolic alterations seen in rats with portacaval shunts could be due to elevated ammonia levels. Furthermore, the synthesis or accumulation of glutamine may be closely linked to cerebral dysfunction in hyperammonaemia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann C., Colombo J. P. Increase of tryptophan and 5-hydroxyindole acetic acid in the brain of ornithine carbamoyltransferase deficient sparse-fur mice. Pediatr Res. 1984 Apr;18(4):372–375. doi: 10.1203/00006450-198404000-00014. [DOI] [PubMed] [Google Scholar]
- Bachmann C., Colombo J. P. Increased tryptophan uptake into the brain in hyperammonemia. Life Sci. 1983 Dec 12;33(24):2417–2424. doi: 10.1016/0024-3205(83)90635-5. [DOI] [PubMed] [Google Scholar]
- Bachmann C., Lüthi H., Gradwohl M., Colombo J. P. Brain uptake of tryptophan in urease-injected hyperammonemic rats after treatment with benzoate or hippurate. Biochem Med Metab Biol. 1986 Oct;36(2):214–219. doi: 10.1016/0885-4505(86)90128-3. [DOI] [PubMed] [Google Scholar]
- Batshaw M. L., Hyman S. L., Mellits E. D., Thomas G. H., DeMuro R., Coyle J. T. Behavioral and neurotransmitter changes in the urease-infused rat: a model of congenital hyperammonemia. Pediatr Res. 1986 Dec;20(12):1310–1315. doi: 10.1203/00006450-198612000-00025. [DOI] [PubMed] [Google Scholar]
- Bircher J. The rat with portacaval shunt: an animal model with chronic hepatic failure. Pharmacol Ther B. 1979;5(1-3):219–222. doi: 10.1016/0163-7258(79)90087-1. [DOI] [PubMed] [Google Scholar]
- Davis D. W., Mans A. M., Biebuyck J. F., Hawkins R. A. The influence of ketamine on regional brain glucose use. Anesthesiology. 1988 Aug;69(2):199–205. doi: 10.1097/00000542-198808000-00008. [DOI] [PubMed] [Google Scholar]
- Diemer N. H., Laursen H. Glial cell reactions in rats with hyperammoniemia induced by urease or porto-caval anastomosis. Acta Neurol Scand. 1977 Jun;55(6):425–442. doi: 10.1111/j.1600-0404.1977.tb07623.x. [DOI] [PubMed] [Google Scholar]
- Eccleston E. G. A method for the estimation of free and total acid-soluble plasma tryptophan using an ultrafiltration technique. Clin Chim Acta. 1973 Oct 30;48(3):269–272. doi: 10.1016/0009-8981(73)90195-2. [DOI] [PubMed] [Google Scholar]
- Eklöf B., Holmin T., Jóhannsson H., Siesjö B. K. Cerebral blood flow and cerebral metabolic rate for oxygen in rats with porta-caval anastomosis. Acta Physiol Scand. 1974 Feb;90(2):337–344. doi: 10.1111/j.1748-1716.1974.tb05595.x. [DOI] [PubMed] [Google Scholar]
- Fernstrom J. D., Wurtman R. J. Brain serotonin content: increase following ingestion of carbohydrate diet. Science. 1971 Dec 3;174(4013):1023–1025. doi: 10.1126/science.174.4013.1023. [DOI] [PubMed] [Google Scholar]
- Fernstrom J. D., Wurtman R. J. Brain serotonin content: physiological regulation by plasma neutral amino acids. Science. 1972 Oct 27;178(4059):414–416. doi: 10.1126/science.178.4059.414. [DOI] [PubMed] [Google Scholar]
- Flynn P. J., Kennan A. L. The rat with a portacaval anastomosis. Arch Pathol. 1968 Feb;85(2):138–148. [PubMed] [Google Scholar]
- Gibson G. E., Zimber A., Krook L., Richardson E. P., Visek W. J. Brain histology and behavior of mice injected with urease. J Neuropathol Exp Neurol. 1974 Apr;33(2):201–211. doi: 10.1097/00005072-197404000-00001. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Mans A. M., Biebuyck J. F. Changes in brain metabolism in hepatic encephalopathy. Neurochem Pathol. 1987 Feb-Apr;6(1-2):35–66. doi: 10.1007/BF02833600. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Mans A. M., Davis D. W., Viña J. R., Hibbard L. S. Cerebral glucose use measured with [14C]glucose labeled in the 1, 2, or 6 position. Am J Physiol. 1985 Jan;248(1 Pt 1):C170–C176. doi: 10.1152/ajpcell.1985.248.1.C170. [DOI] [PubMed] [Google Scholar]
- Herz R., Sauter V., Bircher J. Fortuitous discovery of urate nephrolithiasis in rats subjected to portacaval anastomosis. Experientia. 1972 Jan 15;28(1):27–28. doi: 10.1007/BF01928242. [DOI] [PubMed] [Google Scholar]
- Herz R., Sautter V., Robert F., Bircher J. The Eck fistula rat: definition of an experimental model. Eur J Clin Invest. 1972 Nov;2(6):390–397. doi: 10.1111/j.1365-2362.1972.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Hourani B. T., Hamlin E. M., Reynolds T. B. Cerebrospinal fluid glutamine as a measure of hepatic encephalopathy. Arch Intern Med. 1971 Jun;127(6):1033–1036. [PubMed] [Google Scholar]
- Hoyumpa A. M., Jr, Desmond P. V., Avant G. R., Roberts R. K., Schenker S. Hepatic encephalopathy. Gastroenterology. 1979 Jan;76(1):184–195. [PubMed] [Google Scholar]
- James J. H., Escourrou J., Fischer J. E. Blood-brain neutral amino acid transport activity is increased after portacaval anastomosis. Science. 1978 Jun 23;200(4348):1395–1397. doi: 10.1126/science.663619. [DOI] [PubMed] [Google Scholar]
- James J. H., Hodgman J. M., Funovics J. M., Yoshimura N., Fischer J. E. Brain tryptophan, plasma free tryptophan and distribution of plasma neutral amino acids. Metabolism. 1976 Apr;25(4):471–476. doi: 10.1016/0026-0495(76)90080-9. [DOI] [PubMed] [Google Scholar]
- James J. H., Ziparo V., Jeppsson B., Fischer J. E. Hyperammonaemia, plasma aminoacid imbalance, and blood-brain aminoacid transport: a unified theory of portal-systemic encephalopathy. Lancet. 1979 Oct 13;2(8146):772–775. doi: 10.1016/s0140-6736(79)92119-6. [DOI] [PubMed] [Google Scholar]
- Jeppsson B., James J. H., Edwards L. L., Fischer J. E. Relationship of brain glutamine and brain neutral amino acid concentrations after portacaval anastomosis in rats. Eur J Clin Invest. 1985 Aug;15(4):179–187. doi: 10.1111/j.1365-2362.1985.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Jonung T., Rigotti P., Jeppsson B., James J. H., Peters J. C., Fischer J. E. Methionine sulfoximine prevents the accumulation of large neutral amino acids in brain of hyperammonemic rats. J Surg Res. 1984 Apr;36(4):349–353. doi: 10.1016/0022-4804(84)90110-0. [DOI] [PubMed] [Google Scholar]
- Mans A. M., Biebuyck J. F., Davis D. W., Bryan R. M., Hawkins R. A. Regional cerebral glucose utilization in rats with portacaval anastomosis. J Neurochem. 1983 Apr;40(4):986–991. doi: 10.1111/j.1471-4159.1983.tb08082.x. [DOI] [PubMed] [Google Scholar]
- Mans A. M., Biebuyck J. F., Davis D. W., Hawkins R. A. Portacaval anastomosis: brain and plasma metabolite abnormalities and the effect of nutritional therapy. J Neurochem. 1984 Sep;43(3):697–705. doi: 10.1111/j.1471-4159.1984.tb12789.x. [DOI] [PubMed] [Google Scholar]
- Mans A. M., Biebuyck J. F., Hawkins R. A. Ammonia selectively stimulates neutral amino acid transport across blood-brain barrier. Am J Physiol. 1983 Jul;245(1):C74–C77. doi: 10.1152/ajpcell.1983.245.1.C74. [DOI] [PubMed] [Google Scholar]
- Mans A. M., Biebuyck J. F., Saunders S. J., Kirsch R. E., Hawkins R. A. Tryptophan transport across the blood-brain barrier during acute hepatic failure. J Neurochem. 1979 Aug;33(2):409–418. doi: 10.1111/j.1471-4159.1979.tb05170.x. [DOI] [PubMed] [Google Scholar]
- Mans A. M., Biebuyck J. F., Shelly K., Hawkins R. A. Regional blood-brain barrier permeability to amino acids after portacaval anastomosis. J Neurochem. 1982 Mar;38(3):705–717. doi: 10.1111/j.1471-4159.1982.tb08689.x. [DOI] [PubMed] [Google Scholar]
- Mans A. M., Consevage M. W., DeJoseph M. R., Hawkins R. A. Regional brain monoamines and their metabolites after portacaval shunting. Metab Brain Dis. 1987 Sep;2(3):183–193. doi: 10.1007/BF00999609. [DOI] [PubMed] [Google Scholar]
- Mans A. M., Davis D. W., Biebuyck J. F., Hawkins R. A. Failure of glucose and branched-chain amino acids to normalize brain glucose use in portacaval shunted rats. J Neurochem. 1986 Nov;47(5):1434–1443. doi: 10.1111/j.1471-4159.1986.tb00776.x. [DOI] [PubMed] [Google Scholar]
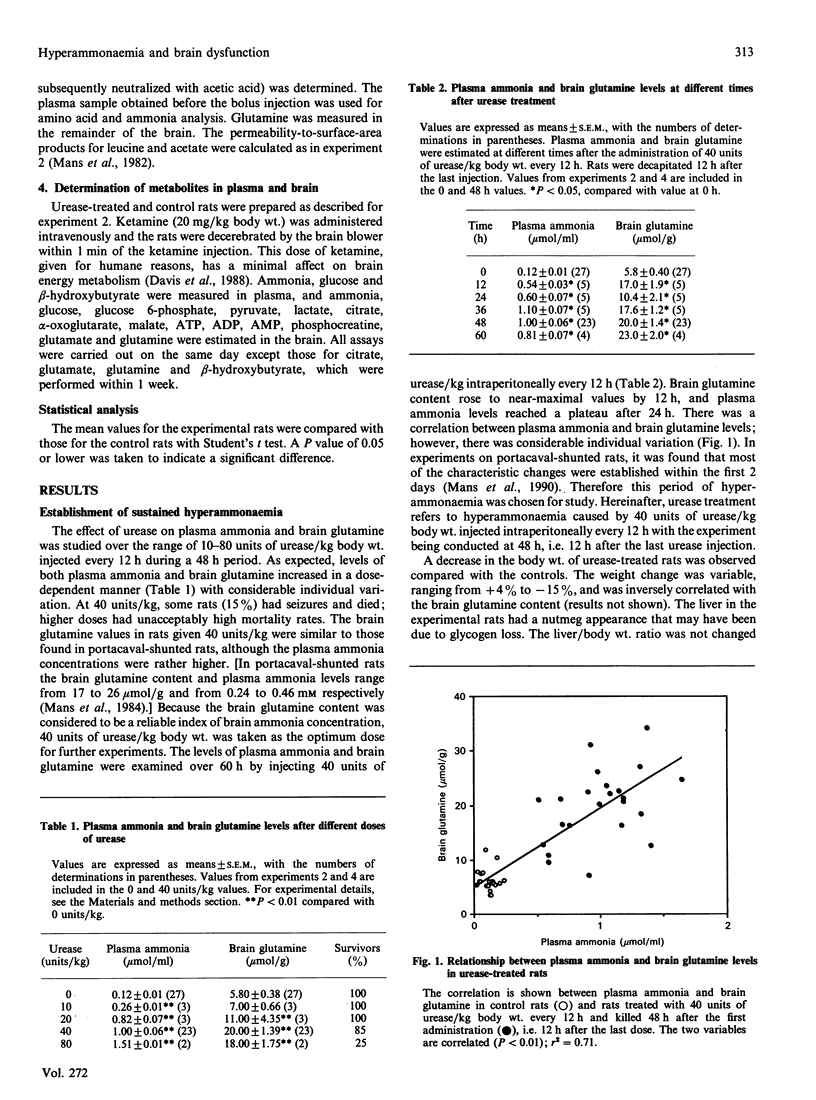
- Mans A. M., DeJoseph M. R., Davis D. W., Viña J. R., Hawkins R. A. Early establishment of cerebral dysfunction after portacaval shunting. Am J Physiol. 1990 Jul;259(1 Pt 1):E104–E110. doi: 10.1152/ajpendo.1990.259.1.E104. [DOI] [PubMed] [Google Scholar]
- Mans A. M., Hawkins R. A. Brain monoamines after portacaval anastomosis. Metab Brain Dis. 1986 Mar;1(1):45–52. doi: 10.1007/BF00998476. [DOI] [PubMed] [Google Scholar]
- Misra P. Hepatic encephalopathy. Med Clin North Am. 1981 Jan;65(1):209–226. doi: 10.1016/s0025-7125(16)31549-8. [DOI] [PubMed] [Google Scholar]
- Nieto C., Arias J., Alsasua A., García de Jalón P. D. Changes in brain oxidative metabolism in rats with portocaval shunt. Experientia. 1980 Dec 15;36(12):1403–1404. doi: 10.1007/BF01960125. [DOI] [PubMed] [Google Scholar]
- Oei L. T., Kuys J., Lombarts A. J., Goor C., Endtz L. J. Cerebrospinal fluid glutamine levels and EEG findings in patients with hepatic encephalopathy. Clin Neurol Neurosurg. 1979;81(1):59–63. doi: 10.1016/s0303-8467(79)80008-6. [DOI] [PubMed] [Google Scholar]
- Prior R. L., Visek W. J. Effects of urea hydrolysis on tissue metabolite concentrations in rats. Am J Physiol. 1972 Nov;223(5):1143–1149. doi: 10.1152/ajplegacy.1972.223.5.1143. [DOI] [PubMed] [Google Scholar]
- Rigotti P., Jonung T., Peters J. C., James J. H., Fischer J. E. Methionine sulfoximine prevents the accumulation of large neutral amino acids in brain of portacaval-shunted rats. J Neurochem. 1985 Mar;44(3):929–933. doi: 10.1111/j.1471-4159.1985.tb12906.x. [DOI] [PubMed] [Google Scholar]
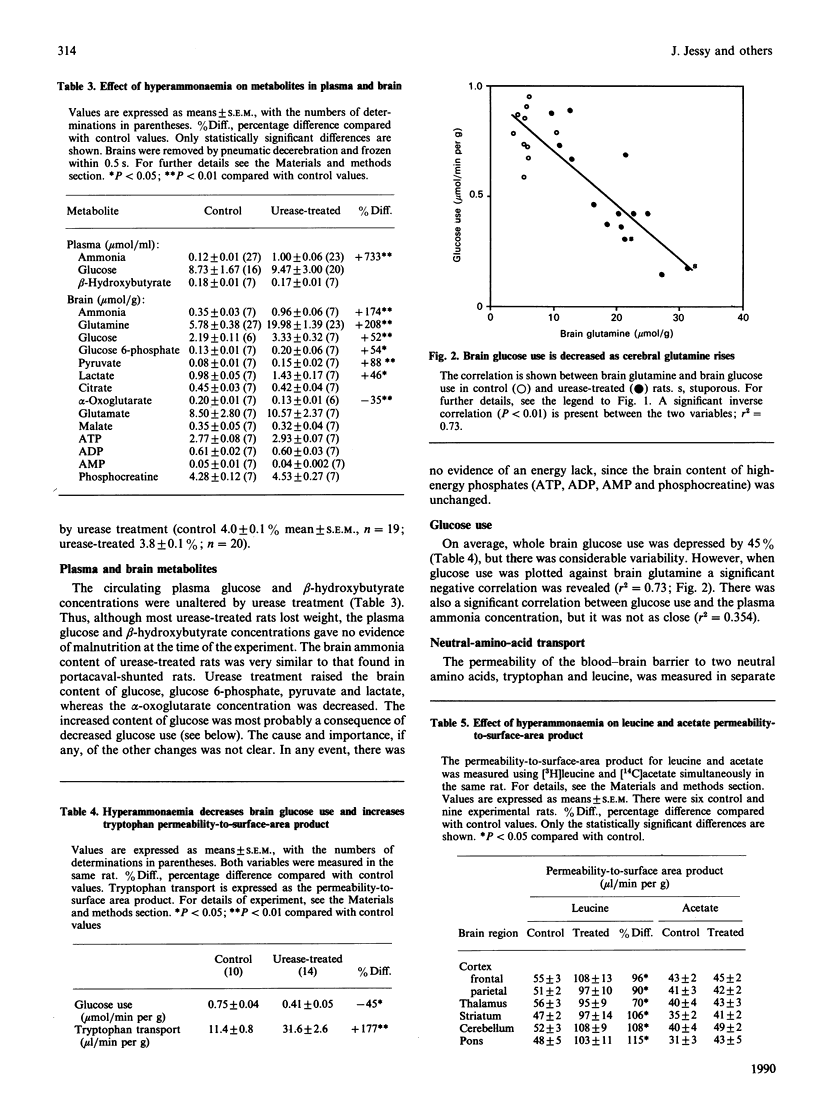
- Sarna G. S., Bradbury M. W., Cremer J. E., Lai J. C., Teal H. M. Brain metabolism and specific transport at the blood-brain barrier after portocaval anastomosis in the rat. Brain Res. 1979 Jan 5;160(1):69–83. doi: 10.1016/0006-8993(79)90601-2. [DOI] [PubMed] [Google Scholar]
- Smith Q. R., Momma S., Aoyagi M., Rapoport S. I. Kinetics of neutral amino acid transport across the blood-brain barrier. J Neurochem. 1987 Nov;49(5):1651–1658. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Zieve L. Hepatic encephalopathy: summary of present knowledge with an elaboration on recent developments. Prog Liver Dis. 1979;6:327–341. [PubMed] [Google Scholar]


