Abstract
Aims
The relationship between serum potassium concentration and outcomes in patients with heart failure and preserved ejection fraction (HFpEF) is not well‐established. The aim of this study was to explore the association between serum potassium and clinical outcomes in the PARAGON‐HF trial in which 4822 patients with HFpEF were randomised to treatment with sacubitril/valsartan or valsartan.
Methods and results
The relationship between serum potassium concentrations and the primary study composite outcome of total (first and recurrent) heart failure hospitalisations and cardiovascular death was analysed. Hypo‐, normo‐, and hyperkalaemia were defined as serum potassium <4 mmol/L, 4–5 mmol/L and >5 mmol/L, respectively. Both screening and time‐updated potassium (categorical and continuous spline‐transformed) were studied. Patient mean age was 73 years and 52% were women. Patients with higher baseline potassium more often had an ischaemic aetiology and diabetes and mineralocorticoid receptor antagonist treatment. Compared with normokalaemia, both time‐updated (but not screening) hypo‐ and hyperkalaemia were associated with a higher risk of the primary outcome [adjusted hazard ratio (HR) for hypokalaemia 1.55, 95% confidence interval (CI) 1.30–1.85; P < 0.001, and for hyperkalaemia HR 1.21, 95% CI 1.02–1.44; P = 0.025]. Hypokalaemia had a stronger association with a higher risk of all‐cause, cardiovascular and non‐cardiovascular death than hyperkalaemia. The association of hypokalaemia with increased risk of all‐cause and cardiovascular death was most marked in participants with impaired kidney function (interaction P < 0.05). Serum potassium did not significantly differ between sacubitril/valsartan and valsartan throughout the follow‐up.
Conclusions
Both hypo‐ and hyperkalaemia were associated with heart failure hospitalisation but only hypokalaemia was associated with mortality, especially in the context of renal impairment. Hypokalaemia was as strongly associated with death from non‐cardiovascular causes as with cardiovascular death. Collectively, these findings suggest that potassium disturbances are a more of a marker of HFpEF severity rather than a direct cause of death.
Keywords: Serum potassium, Heart failure with preserved ejection fraction, Outcomes, Sacubitril/valsartan
Introduction
Both hypo‐ and hyperkalaemia are common in heart failure with preserved ejection fraction (HFpEF). Both incident hypokalaemia and hyperkalaemia are associated with a worse prognosis with a U‐shaped association between potassium and mortality. 1 , 2 , 3 , 4 Patients with HFpEF are often elderly with concomitant chronic kidney disease (CKD), which may increase the risk for abnormalities in potassium homeostasis during treatment. 5 Moreover, some commonly used medications [e.g. loop and thiazide‐type diuretics, mineralocorticoid receptors antagonists (MRAs), angiotensin‐converting enzyme inhibitors (ACEi)/angiotensin receptor blockers (ARBs), and beta‐blockers] may contribute to dyskalaemia. 6
Both severe hypo‐ and hyperkalaemia may increase the risk of arrhythmias and sudden cardiac death, especially if these perturbations develop rapidly and are marked (e.g. serum potassium <2 mmol/L or >7 mmol/L). 7 , 8 , 9 The reason why less extreme potassium abnormalities are associated with poor outcomes is uncertain, and these may just be a marker of HFpEF severity (or associated comorbidities), rather than a direct cause of death. In patients with heart failure and reduced ejection fraction (HFrEF) the association between higher potassium concentrations and worse outcomes has been blamed on the withdrawal of renin–angiotensin–aldosterone system (RAAS) blockers but such a mechanism is less plausible in HFpEF where the benefit of RAAS blockers is uncertain. 10 , 11 , 12 , 13 , 14 Conversely, in both heart failure phenotypes, hypokalaemia may reflect intensity of diuresis, in turn representing severity of heart failure. 5 , 15 , 16 , 17 , 18 Compared to HFrEF, fewer studies have examined the relationship between serum potassium and outcomes in HFpEF. Moreover, the incidence of both hypo‐ and hyperkalaemia with sacubitril/valsartan vs. valsartan has not been documented yet. Therefore, we studied the efficacy and safety of sacubitril/valsartan, compared to valsartan, in 4822 patients with HFpEF in PARAGON‐HF (Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction). 19
We examined the relationships between serum potassium concentration (both categorical and continuous) and outcomes in patients with HFpEF enrolled in PARAGON‐HF and the potential interaction with renal function. We also studied the effect of trial treatment on the incidence of hyperkalaemia and hypokalaemia throughout the study follow‐up.
Methods
Study design and population
The design and primary results of PARAGON‐HF are published. 19 , 20 Briefly, patients aged ≥50 years were eligible if they had New York Heart Association functional class II–IV symptoms, preserved left ventricular ejection fraction (LVEF ≥45%), additional evidence of structural heart disease (left ventricular hypertrophy, left atrial enlargement, or both), need for diuretic therapy and elevated levels of natriuretic peptides. Key exclusion criteria included any prior LVEF <40%, acute decompensated heart failure at the time of screening, symptomatic hypotension (or systolic blood pressure <100 mmHg at screening), estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2, or serum potassium >5.2 mmol/L at screening.
Patients undertook sequential valsartan and sacubitril/valsartan run‐in periods before randomisation. During the 1‐ to 2‐week valsartan run‐in, valsartan 40 mg or 80 mg was administered twice daily; patients receiving the lower dose initially were increased to 80 mg twice daily. Patients tolerating valsartan were then exposed to a further 2‐ to 4‐week run‐in period during which they received sacubitril/valsartan 49/51 mg twice daily. Only patients who tolerated both study drugs were eligible for randomisation. At randomisation, doses were increased to sacubitril/valsartan 97/103 mg twice daily or valsartan 160 mg twice daily when possible.
The ethics committee at each participating site approved the study protocol, and participants provided written informed consent. The trial is registered in ClinicalTrials.gov with the number NCT01920711.
Study treatment, follow‐up and laboratory measurements
Serum potassium and creatinine levels were measured at screening, during the run‐in period, at randomisation, 1 and 4 months following randomisation, and in 4‐month intervals thereafter at each study site. eGFR was calculated using the Modification of Diet in Renal Disease formula. 21
By protocol, a patient with a serum potassium >5.3 mmol/L at any time after randomisation required confirmation in a non‐haemolysed sample and further checks of potassium concentration until the potassium was stable and not rising into the range of concern (≥5.5 and <6.0 mmol/L) or potential danger (≥6.0 mmol/L). Guidance was given on modification of trial treatment (including dose reduction or discontinuation), concomitant therapy and diet, to correct elevation in potassium. Hypokalaemia was managed at the discretion of the treating physician. 19
Endpoints
In the present analysis we studied the associations of serum potassium (categorical and continuous, using both the screening and time‐updated values) with: (i) the trial primary endpoint, a composite of total (first and recurrent) heart failure hospitalisations and cardiovascular death; (ii) total heart failure hospitalisations; (iii) cardiovascular death; (iv) non‐cardiovascular death; and (v) all‐cause death. There were too few sudden cardiac deaths and ‘pump failure’ deaths for meaningful analysis.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation or median (percentile25–75). Categorical variables are presented as absolute numbers and percentages. The baseline characteristics were compared between the following potassium categories of <4 (hypokalaemia), 4–5 (normokalaemia), and >5 mmol/L (hyperkalaemia) at screening, using ANOVA or Kruskal–Wallis tests as appropriate. The variables with strongest association with hypo‐ and hyperkalaemia during the follow‐up were assessed using a stepwise forward multinomial logistic model with normokalaemia set as reference category and all the variables with a P‐value <0.1 from Table 1 included in the model as independent variables. Several models were used to study the association of serum potassium with the outcomes. The associations between serum potassium at screening and the outcomes of interest were studied using Cox models with serum potassium as both a categorical and a continuous variable (using restricted cubic splines 22 ). Repeated potassium measurements throughout the follow‐up period were incorporated in time‐updated models (that take into account the last available potassium value before each event), also examining potassium as a categorical and a continuous variable (spline transformed). For the composite of total (first and recurrent) heart failure hospitalisations and cardiovascular death, as well as total heart failure hospitalisations, a semiparametric proportional rates method (LWYY) and a joint gamma frailty model stratified according to geographic region was used, in concordance with the primary report of the trial. 19 The regression estimates are presented as hazard ratios (HR) with 95% confidence intervals (CIs). The adjusted models were corrected for age, sex, race, region, systolic blood pressure, heart rate, body mass index, eGFR (categorical: <60 vs. ≥60 mL/min/1.73 m2), blood urea nitrogen, ischaemic cardiomyopathy, LVEF, N‐terminal pro‐B‐type natriuretic peptide, New York Heart Association (NYHA) class, hypertension, diabetes, atrial fibrillation, prior heart failure hospitalisation, prior stroke, use of diuretics (non‐MRAs), beta‐blockers, MRAs, and treatment group allocation (sacubitril/valsartan or valsartan) at randomisation. In the time‐updated models, we pre‐specified two interaction terms, one with potassium by eGFR (fitted as a time‐updated covariate) and another with potassium by treatment (sacubitril/valsartan vs. valsartan) to assess whether the prognostic associations of serum potassium could change by eGFR or by treatment, respectively. The rationale for testing the interaction by renal function and treatment allocation was based on the important impact of renal function on both the prognostic and potassium levels in heart failure 23 and testing sacubitril/valsartan vs. valsartan in HFpEF was the main aim of the PARAGON‐HF trial. Whenever a statistically significant interaction was found, the models were presented separately in the respective subgroups. An exploratory landmark analysis studying the associations between the number of hypo‐ and hyperkalaemia episodes (0, 1 and 2 or more) plus the average potassium levels during the first 8 months of the trial and subsequent primary outcome events (to preserve some power for this analysis) was also performed. Mixed‐effects models were used to analyse changes in serum potassium concentration over time, according to randomised treatment group and whether patients were treated with a MRA or not; a treatment‐by‐visit time interaction term was forced in the model as fixed effect and the random effects were set at the patient level. All statistical analyses were conducted in Stata® version 16 (StataCorp LP, College Station, TX, USA). A P‐value <0.05 was accepted as the threshold for statistical significance without correction for multiplicity of tests given the exploratory nature of this analysis.
Table 1.
Characteristics of the population by potassium categories at screening in the PARAGON‐HF trial
| Characteristics | K+ <4 (n = 592) | K+ 4–5 (n = 3877) | K+ >5 (n = 327) | P‐value |
|---|---|---|---|---|
| Age, years | 73 ± 9 | 73 ± 8 | 73 ± 8 | 0.68 |
| Male sex | 260 (43.9%) | 1895 (48.9%) | 162 (49.5%) | 0.07 |
| Race | 0.001 | |||
| White | 444 (75.0%) | 3170 (81.8%) | 293 (89.6%) | |
| Asian | 96 (16.2%) | 485 (12.5%) | 26 (8.0%) | |
| Black | 29 (4.9%) | 71 (1.8%) | 2 (0.6%) | |
| Other | 23 (3.9%) | 151 (3.9%) | 6 (1.8%) | |
| Region | 0.001 | |||
| North America | 114 (19.3%) | 419 (10.8%) | 26 (8.0%) | |
| Latin America | 59 (10.0%) | 294 (7.6%) | 17 (5.2%) | |
| Western Europe | 178 (30.1%) | 1113 (28.7%) | 99 (30.3%) | |
| Central Europe | 128 (21.6%) | 1441 (37.2%) | 146 (44.6%) | |
| Asia‐Pacific or other | 113 (19.1%) | 610 (15.7%) | 39 (11.9%) | |
| SBP, mmHg | 132 ± 16 | 130 ± 15 | 130 ± 15 | 0.09 |
| Heart rate, bpm | 70 ± 13 | 70 ± 12 | 71 ± 12 | 0.98 |
| BMI, kg/m2 | 30.2 ± 5.4 | 30.2 ± 5.0 | 30.2 ± 4.8 | 0.98 |
| eGFR, mL/min/1.73 m2 | 63.5 ± 19.3 | 62.6 ± 19.0 | 60.4 ± 19.5 | 0.05 |
| eGFR <60 mL/min/1.73 m2 | 279 (47.1%) | 1886 (48.6%) | 176 (53.8%) | 0.14 |
| BUN, mmol/L | 7.74 ± 3.37 | 8.10 ± 3.17 | 8.79 ± 3.72 | 0.001 |
| Ischaemic HF | 176 (29.7%) | 1417 (36.5%) | 130 (39.8%) | 0.002 |
| LVEF, % | 58.0 ± 7.7 | 57.5 ± 7.9 | 57.3 ± 8.3 | 0.22 |
| NYHA class | 0.79 | |||
| I | 18 (3.0%) | 106 (2.7%) | 13 (4.0%) | |
| II | 452 (76.4%) | 3011 (77.7%) | 245 (74.9%) | |
| III | 119 (20.1%) | 746 (19.2%) | 67 (20.5%) | |
| IV | 3 (0.5%) | 14 (0.4%) | 2 (0.6%) | |
| Hypertension | 569 (96.1%) | 3705 (95.6%) | 310 (94.8%) | 0.65 |
| Diabetes | 246 (41.6%) | 1645 (42.4%) | 171 (52.3%) | 0.002 |
| Atrial fibrillation | 210 (35.5%) | 1243 (32.1%) | 99 (30.3%) | 0.18 |
| Prior HF hospitalisation | 286 (48.3%) | 1849 (47.7%) | 171 (52.3%) | 0.28 |
| MI | 124 (20.9%) | 884 (22.8%) | 75 (22.9%) | 0.6 |
| Stroke | 77 (13.0%) | 399 (10.3%) | 32 (9.8%) | 0.12 |
| Prior ACEi | 217 (36.7%) | 1632 (42.1%) | 132 (40.4%) | 0.041 |
| Prior ARB | 282 (47.6%) | 1799 (46.4%) | 168 (51.4%) | 0.21 |
| Diuretic | 565 (95.4%) | 3703 (95.5%) | 317 (96.9%) | 0.47 |
| Beta‐blocker | 470 (79.4%) | 3090 (79.7%) | 261 (79.8%) | 0.98 |
| MRA | 130 (22.0%) | 1004 (25.9%) | 105 (32.1%) | 0.003 |
| NT‐proBNP, pg/mL | 979 [484–1847] | 901 [465–1562] | 885 [441–1747] | 0.06 |
| Sacubitril/valsartan | 274 (46.3%) | 1968 (50.8%) | 165 (50.5%) | 0.13 |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; HF, heart failure; K+, serum potassium (mmol/L); LVEF, left ventricular ejection fraction; MI, myocardial infraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure.
Results
Baseline characteristics and variables associated with hypo‐ and hyperkalaemia
Baseline characteristics of patients in PARAGON‐HF, according to serum potassium levels at screening (<4 vs. 4–5 vs. >5 mmol/L), are shown in Table 1 . The were no significant differences with regard to age, sex, NYHA class, or ejection fraction by potassium category. Patients with potassium <4 mmol/L were more often from Asia‐Pacific, North and Latin America, whereas those with potassium >5 mmol/L were more often from Central Europe. Patients with heart failure of ischaemic aetiology, diabetes and those treated with an MRA had higher potassium levels at screening.
Throughout the follow‐up a blood urea nitrogen >8 mmol/L, Central Europe region, White race, and diabetes were associated with incident hyperkalaemia at multiple visits, whereas high blood pressure/hypertension history and the use of diuretics were associated with incident hypokalaemia. Randomised assignment to sacubitril/valsartan was not associated with either hypo‐ or hyperkalaemia throughout the follow‐up. Non‐randomised use of MRAs was associated with incident hyperkalaemia at week 4 and week 32 only (online supplementary Table S1 ).
Post‐hoc tests for the results displayed in Table 1 are presented in online supplementary Table S2 .
Clinical outcomes
Event rates related to screening potassium category, and time‐updated potassium, are presented in Table 2 . Patients in the lowest and highest time‐updated potassium categories had a higher risk of the primary outcome than those with a potassium between 4 and 5 mmol/L. With normokalaemia (4–5 mmol/L) used as the reference category, the adjusted time‐updated HR (95% CI) for the primary outcome in patients with incident hypokalaemia (potassium <4 mmol/L) was 1.55 (1.30–1.85) (P < 0.001) and for patients with incident hyperkalaemia (potassium >5 mmol/L) the adjusted time‐updated HR was 1.21 (1.02–1.44) (P = 0.025). The relationship between serum potassium as a continuous (spline transformed) variable and the primary outcome, suggests that potassium levels above 5.0 mmol/L and below 4.5 mmol/L are each associated with a higher risk of the primary outcome (Figure 1 ). Similar findings were observed for total heart failure hospitalisations alone. Incident hypokalaemia had a stronger association with cardiovascular death than incident hyperkalaemia. Similarly, hypokalaemia had a stronger association with non‐cardiovascular death [adjusted time‐updated HR (95% CI) 1.72 (1.20–2.48); P = 0.003 and 0.93 (0.61–1.40); P = 0.71, for hypo‐ and hyperkalaemia, respectively]. As a result, hypokalaemia, but not hyperkalaemia, was strongly associated with death from any cause.
Table 2.
Events, event rates, and hazard ratios for the screening and time‐updated potassium categories for key study outcomes in the PARAGON‐HF trial
| K+ (mmol/L) | Events, n (%) | Incidence rate per 100 py | Screening model (crude) | Screening model (adjusted) | P‐value * | Time‐updated model (crude) | Time‐updated model (adjusted) | P‐value * | K+ by eGFR interaction P‐value ** |
|---|---|---|---|---|---|---|---|---|---|
| Primary outcome | |||||||||
| <4 | 276 (33.8) | 16.0 (13.0–20.0) | 1.06 (0.84–1.33) | 1.03 (0.83–1.30) | 0.77 | 1.55 (1.30–1.85) | 1.55 (1.30–1.85) | <0.001 | |
| 4–5 | 1466 (29.3) | 13.1 (12.1–14.2) | Referent | Referent | – | Referent | Referent | – | 0.46 |
| >5 | 161 (35.4) | 17.2 (13.3–22.7) | 1.42 (1.08–1.87) | 1.29 (0.99–1.69) | 0.058 | 1.60 (1.35–1.88) | 1.21 (1.02–1.44) | 0.025 | |
| Total HF hospitalisations | |||||||||
| <4 | 225 (27.5) | 13.1 (10.3–16.8) | 1.09 (0.84–1.40) | 1.03 (0.81–1.32) | 0.79 | 1.64 (1.24–2.00) | 1.61 (1.33–1.96) | <0.001 | |
| 4–5 | 1134 (22.6) | 10.1 (9.2–11.1) | Referent | Referent | – | Referent | Referent | – | 0.67 |
| >5 | 128 (28.1) | 13.7 (10.2–18.8) | 1.48 (1.08–2.03) | 1.34 (0.99–1.81) | 0.060 | 1.64 (1.36–1.97) | 1.26 (1.04–1.52) | 0.019 | |
| CV death | |||||||||
| <4 | 51 (8.6) | 3.0 (2.2–3.9) | 1.00 (0.74–1.34) | 1.01 (0.75–1.37) | 0.93 | 1.37 (1.03–1.81) | 1.42 (1.06–1.89) | 0.018 | |
| 4–5 | 332 (8.6) | 3.0 (2.7–3.3) | Referent | Referent | – | Referent | Referent | – | 0.004 |
| >5 | 33 (10.1) | 3.5 (2.5–5.0) | 1.19 (0.83–1.70) | 1.13 (0.79–1.62) | 0.51 | 1.56 (1.20–2.03) | 1.16 (0.88–1.52) | 0.29 | |
| All‐cause death | |||||||||
| <4 | 82 (13.9) | 4.8 (3.8–5.9) | 0.96 (0.76–1.21) | 0.99 (0.78–1.25) | 0.90 | 1.46 (1.17–1.80) | 1.51 (1.21–1.87) | <0.001 | |
| 4–5 | 554 (14.3) | 4.9 (4.6–5.4) | Referent | Referent | – | Referent | Referent | – | 0.042 |
| >5 | 55 (16.8) | 5.9 (4.5–7.6) | 1.19 (0.90–1.57) | 1.12 (0.85–1.48) | 0.43 | 1.39 (1.13–1.72) | 1.06 (0.85–1.32) | 0.61 | |
| Non‐CV death | |||||||||
| <4 | 29 (4.9) | 1.7 (1.2–2.4) | 1.07 (0.72–1.59) | 1.09 (0.73–1.62) | 0.69 | 1.69 (1.18–2.42) | 1.72 (1.20–2.48) | 0.003 | |
| 4–5 | 175 (4.5) | 1.6 (1.3–1.8) | Referent | Referent | – | Referent | Referent | – | 0.28 |
| >5 | 16 (4.9) | 1.7 (1.0–2.8) | 1.10 (0.66–1.84) | 1.03 (0.61–1.72) | 0.92 | 1.13 (0.75–1.69) | 0.93 (0.61–1.40) | 0.71 |
CV, cardiovascular; eGFR, estimated glomerular filtration rate (mL/min/1.73 m2); HF, heart failure; K+, serum potassium (mmol/L); py, patient‐years.
Notes: The interaction P of K+ by eGFR (<60 or ≥60 mL/min/1.73 m2) and treatment (sacubitril/valsartan or valsartan) was assessed in the fully adjusted model. The P for interaction with eGFR is presented in the table. The P for interaction with treatment was non‐significant for all comparisons, as follows: primary outcome =0.96; total HF hospitalisations =0.96; CV death =0.25; all‐cause death =0.13; non‐CV death =0.31.
All models are adjusted for age, sex, race, region, systolic blood pressure, heart rate, body mass index, eGFR (categorical: <60 vs. ≥60 mL/min/1.73 m2), blood urea nitrogen, ischaemic cardiomyopathy, left ventricular ejection fraction, N‐terminal pro‐B‐type natriuretic peptide, New York Heart Association class, hypertension, diabetes, atrial fibrillation, prior HF hospitalisation, prior stroke, use of diuretics, beta‐blockers, mineralocorticoid receptor antagonists, and treatment group allocation (sacubitril/valsartan or valsartan).
P‐value for the fully adjusted model.
Interaction P‐value for the fully adjusted time‐updated model. Significant interactions are presented in Table 3 , separated by eGFR subgroups.
Figure 1.
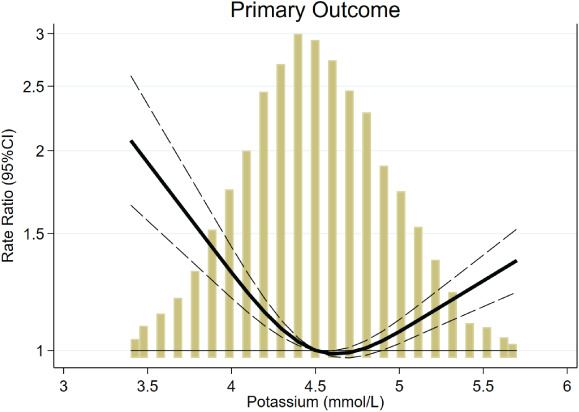
Association of time‐updated serum potassium with the study primary outcome. The primary outcome was a composite of total (first and recurrent) heart failure hospitalisations and cardiovascular death. Both hypo‐ and hyperkalaemia were associated with the study primary outcome. Yellow bars, histogram of time‐updated potassium. CI, confidence interval.
In an exploratory landmark analysis, patients with two or more episodes of hypokalaemia had a higher subsequent risk of the primary outcome [HR (95% CI) 1.32 (1.07–1.62); P = 0.009 and 1.20 (0.98–1.47); P = 0.08, for hypo‐ and hyperkalaemia, respectively] (online supplementary Table S3 ). Averaging all potassium measurements during the first 8 months of the trial showed that hypokalaemia had a stronger association with subsequent primary endpoints than hyperkalaemia [HR (95% CI) 1.35 (1.03–1.75); P = 0.028 and 1.22 (0.95–1.57); P = 0.12, for hypo‐ and hyperkalaemia, respectively; online supplementary Table S3 ].
The associations reported in Table 2 remained similar when an adjustment was made for time‐updated use of diuretics, reinforcing the stronger association of hypokalaemia (compared with hyperkalaemia) with worse outcomes (online supplementary Table S4 ).
Serum potassium by renal function and treatment interaction
A significant time‐updated serum potassium‐by‐kidney function (eGFR <60 vs. ≥60 mL/min/1.73 m2) interaction was found for the outcomes of cardiovascular death and all‐cause death (interaction P <0.05 for both) as shown in the Table 3 . Patients with impaired renal function had higher risk of both cardiovascular and non‐cardiovascular death in the presence of hypokalaemia, but not in the presence of hyperkalaemia, whereas patients with normal renal function experienced no excess of risk related to either type of potassium perturbation (Table 3 ). These findings are expanded in Figure 2 where we can see that patients with an eGFR <60 mL/min/1.73 m2 had a higher risk of mortality than patients with an eGFR ≥60 mL/min/1.73 m2 regardless of the potassium level; however, the mortality risk is particularly high (by more than twofold) among patients with impaired renal function and potassium levels <4 mmol/L. On the other hand, the risk of death is not influenced by potassium levels among patients with an eGFR ≥60 mL/min/1.73 m2. Analyses with further eGFR subgrouping are shown in online supplementary Table S5 .
Table 3.
Significant interactions of time‐updated potassium categories by time‐updated estimated glomerular filtration rate
| Outcome/eGFR subgroup | HR (95% CI) | P‐value | eGFR by K+ interaction P‐value |
|---|---|---|---|
| CV death | |||
| K+ in eGFR ≥60 | |||
| <4 | 0.65 (0.34–1.22) | 0.18 | |
| 4–5 | Referent | – | |
| >5 | 0.81 (0.42–1.57) | 0.54 | |
| K+ in eGFR <60 | 0.004 | ||
| <4 | 1.98 (1.42–2.75) | <0.001 | |
| 4–5 | Referent | – | |
| >5 | 1.28 (0.94–1.73) | 0.11 | |
| All‐cause death | |||
| K+ in eGFR ≥60 | |||
| <4 | 0.94 (0.62–1.44) | 0.77 | |
| 4–5 | Referent | – | |
| >5 | 1.10 (0.69–1.74) | 0.69 | |
| K+ in eGFR <60 | 0.042 | ||
| <4 | 1.88 (1.45–2.43) | <0.001 | |
| 4–5 | Referent | – | |
| >5 | 1.06 (0.82–1.35) | 0.67 |
CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate (mL/min/1.73 m2); HR, hazard ratio; K+, serum potassium (mmol/L).
Figure 2.
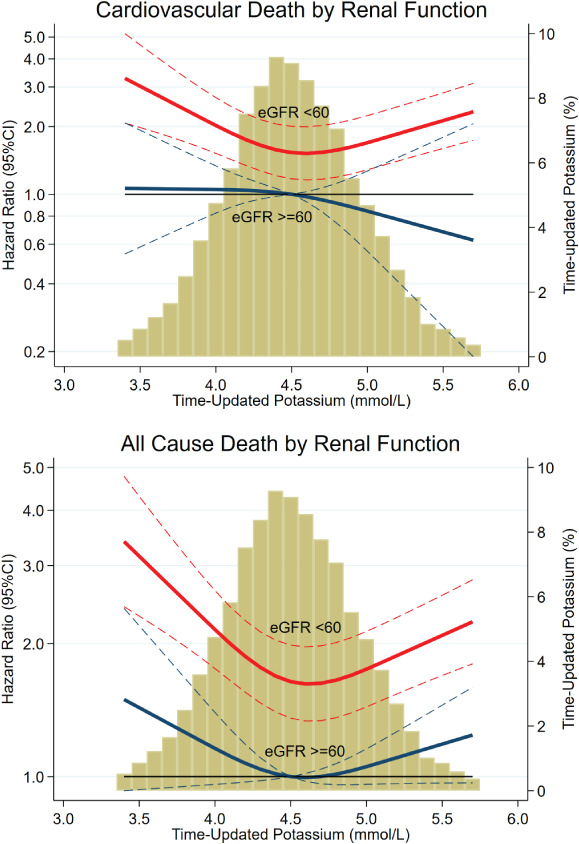
Time‐updated serum potassium‐by‐renal function interaction. eGFR, estimated glomerular filtration rate (mL/min/1.73 m2). Patients with an eGFR <60 mL/min/1.73 m2 had a higher mortality risk than patients with eGFR ≥60 mL/min/1.73 m2, regardless of potassium level. However, the risk is particularly elevated (by more than twofold) when both an eGFR <60 mL/min/1.73m2 and a potassium <4.0 mmol/L were present; P for interaction <0.05 for both outcomes. Yellow bars, histogram of time‐updated potassium. CI, confidence interval.
The effect of sacubitril/valsartan, compared with valsartan, was not modified by baseline potassium levels: treatment‐by‐potassium P for interaction for the primary outcome =0.96; total heart failure hospitalisations =0.96; cardiovascular death =0.25; all‐cause death =0.13; non‐cardiovascular death =0.31 (Table 2 ).
Serum potassium levels over time
No significant differences in the level of serum potassium were found between the sacubitril/valsartan and valsartan groups, irrespective of background use of an MRA (Figure 3 ).
Figure 3.
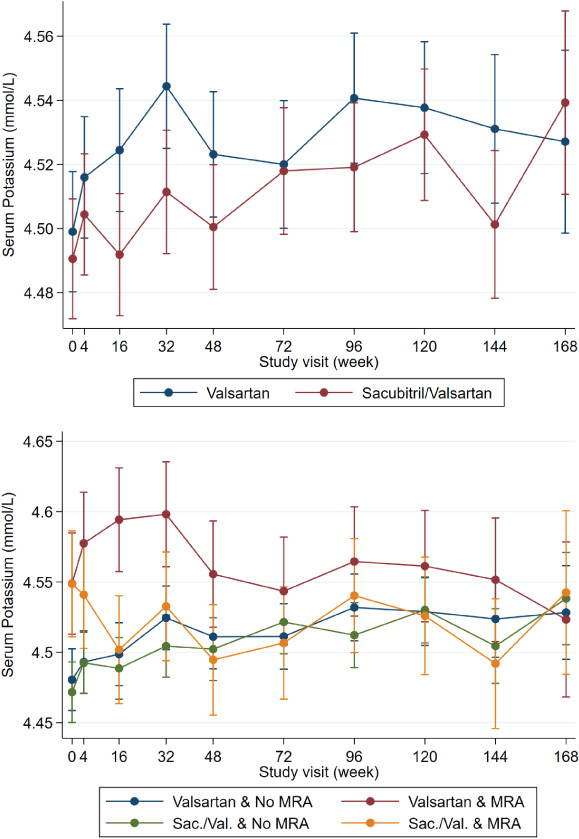
Potassium levels over time by treatment group and mineralocorticoid receptor antagonist (MRA) treatment. No significant between‐group differences were present.
Discussion
This analysis showed that in patients with HFpEF, a serum potassium <4 mmol/L or >5 mmol/L was associated with higher rates of hospitalisation for heart failure than observed in patients with a potassium between 4 and 5 mmol/L. The elevation in risk was more marked for hypokalaemia than for hyperkalaemia. The risk of death was also higher in patients with hypokalaemia, but not in patients with hyperkalaemia. Furthermore, there was an interaction between potassium and kidney function, whereby the elevated risk of death related to hypokalaemia was only observed in patients with impaired kidney function (eGFR <60 mL/min/1.73 m2). The effect of sacubitril/valsartan was not modified by serum potassium and the mean potassium levels throughout follow‐up did not differ significantly between the sacubitril/valsartan and valsartan groups.
The association between serum potassium and clinical outcomes has been documented less often in HFpEF than in HFrEF, 23 although the few studies that have examined this question in HFpEF have described a U‐shaped relationship between potassium and mortality, similar to that seen in HFrEF. 1 , 2 , 24 However, the studies to date have focussed on all‐cause and cardiovascular death, and the elevated risk of death has been attributed to the occurrence of fatal ventricular arrhythmias, related to disturbances in potassium. However, our finding that hypo‐ and hyperkalaemia were each associated with heart failure hospitalisation questions this postulated mechanism, as does the association between hypokalaemia and non‐cardiovascular death. There is no clear, direct, mechanism by which hypo‐ or hyperkalaemia should be associated with either of these outcomes, suggesting that potassium perturbations in most cases are more likely markers rather than mediators of risk.
The stronger relationship between hypokalaemia and adverse outcomes, compared with hyperkalaemia, may reflect the specific exclusion of patients with an elevated potassium at screening and during the run‐in phases in PARAGON‐HF, and the protocol‐mandated monitoring for and correction of hyperkalaemia (there were no specific recommendations for management of hypokalaemia in the protocol).
The interaction between hypokalaemia and renal impairment in relation to fatal outcomes is of interest. An association between eGFR and hyperkalaemia might have been expected more than an association with hypokalaemia. We think the unexpected association between low eGFR and hypokalaemia (rather than hyperkalaemia) may reflect the intensity of non‐MRA diuretic use and the less frequent use of MRAs, with a notable difference in prescription of the latter across the three potassium categories (hypokalaemia 22.0%, normokalaemia 26% and hyperkalaemia 32%). 14 , 25 Given the findings of the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist study (TOPCAT), suggesting therapy with spironolactone may benefit at least some patients with HFpEF, 14 MRAs could be considered in this population as they also decrease hypokalaemia, which is strongly associated with adverse outcomes. Furthermore, PARAGON‐HF patients treated with sacubitril/valsartan and MRAs had a less pronounced annualised eGFR decline and did not experience excess adverse events including severe hyperkalaemia (potassium >6.0 mmol/L), which further support the concomitant use of MRAs with sacubitril/valsartan. 26
Hypokalaemia and kidney impairment have been associated with higher use of diuretics, lower use of RAAS inhibitors (including MRAs), and malnutrition, 27 all of which may contribute to poor outcomes. Furthermore, models adjusted for time‐updated use of diuretics reinforced the association between hypokalaemia (and not hyperkalaemia) with worse outcomes, supporting the hypothesis that the potassium alterations (hypokalaemia in this case) are more of a marker of worse symptoms and HFpEF severity that can arise as a consequence of diuretic therapy intensification, rather than a direct cause of events. It should also be noted that, in time‐updated models, it is the last potassium value registered before the event that is considered, supporting the prompt correction of hypokalaemia (as soon as detected) regardless of the cause or course.
Our findings differ somewhat from those in patients with HFrEF in the Prospective Comparison of ARNI with an ACE‐Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure trial (PARADIGM‐HF). 28 In that trial, both hyper‐ and hypokalaemia were independently associated with heart failure hospitalisation, again suggesting that potassium abnormalities are mainly markers of disease severity. However, both high as well as low potassium were associated with death (including non‐cardiovascular death) in PARADIGM‐HF and there was no interaction with renal function. The explanation for these differences is uncertain, but may be due to difference in patient characteristics in the two trials, with participants in PARAGON‐HF considerably older than in PARADIGM‐HF (mean age 73 vs. 64 years) and with worse renal function (mean eGFR 63 vs. 68 mL/min/1.73 m2).
In PARAGON‐HF, sacubitril/valsartan did not reduce the mean serum potassium compared with valsartan, which was also different than in PARADIGM‐HF where, sacubitril/valsartan reduced mean serum potassium concentration compared with enalapril. This difference may again be due to the different study populations or in the active comparator. However, in both trials sacubitril/valsartan reduced the incidence of significant hyperkalaemia, i.e. a potassium >6 mmol/L. 19 , 29
Limitations
This was a post‐hoc analysis of the PARAGON‐HF trial. Patients had to tolerate recommended doses of valsartan and sacubitril/valsartan, which may reduce the generalisability of our findings. Regular monitoring of potassium and protocol‐guided mitigation might have led to a prompt correction of potassium abnormalities which may not occur in routine practice. Serum potassium level >5.2 mmol/L at screening (or >5.4 mmol/L at randomisation) was an exclusion criterion, which might have led to the selection of patients less prone to develop hyperkalaemia and the protocol included recommendations to manage hyperkalaemia, both of which may have reduced the impact of hyperkalaemia relative to hypokalaemia in the trial. However, as potassium changes over time, the time‐updated analyses were more informative because they accounted for the last potassium value available before the event. Routine laboratory analyses, including potassium, were performed at local laboratories and some cases of haemolysis might have occurred. Diuretic doses are not standardised and are inconsistently reported as ‘free text’ in the dataset, which would lead to unreliable data; therefore, we did not adjust for the dose of diuretics in our models. We do not report data on non‐fatal arrhythmias because these were not systematically recorded in the dataset. The use of potassium supplements or binders was not systematically recorded in the trial. The proportion of patients with potassium levels <3.5 mmol/L and >5.5 mmol/L was small; hence, these findings cannot be generalised to patients with either very low or very high potassium levels.
Conclusions
Both hypo‐ and hyperkalaemia were associated with heart failure hospitalisations but only hypokalaemia was associated with mortality, especially in the context of renal impairment. Hypokalaemia was as strongly associated with death from non‐cardiovascular causes as with cardiovascular death. Collectively, these findings suggest that potassium disturbances are a more of a marker of HFpEF severity rather than a direct cause of death.
Funding
J.P.F. is funded by an ESC research grant for collaboration with the University of Glasgow. All other authors report no specific funding for this project. J.J.V.McM. and P.S.J. are supported by a British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217.
Conflict of interest: S.J.S. has received research grants from Actelion, AstraZeneca, Corvia, Novartis, and Pfizer; and has received consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Axon Therapies, Bayer, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Cardiora, CVRx, Cytokinetics, Eisai, GSK, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Sanofi, Shifamed, Tenax, and United Therapeutics. J.G.C. has received research grants from Bayer, Bristol‐Myers Squibb, Pharmacosmos and Vifor and has received honoraria for advisory boards, lectures and or trial committees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer‐Ingelheim, Bristol‐Myers Squibb, HeartFelt Technologies, Idorsia, Johnson & Johnson, Medtronic, Myokardia, Novartis, Pharmacosmos, Respicardia, Servier, Torrent, Vifor and Viscardia. B.L.C., A.S.D., M.A.P., I.S.A., D.J.V.V., L.K., J.G.F.C., B.P., J.L.R., M.P., M.R.Z., V.C.S., M.P.L., S.J.S., O.V., F.Z., and J.J.V.McM. are steering committee members of the PARAGON‐HF trial. All other authors have nothing to disclose.
Supporting information
Table S1. Predictors of hypo‐ and hyperkalaemia throughout the follow‐up.
Table S2. Pairwise comparison for variables with significant differences in Table 1 .
Table S3. Landmark analysis assessing the impact of the number of hypo‐ and hyperkalaemia episodes and the average potassium levels during the first 8 months of the trial and subsequent primary outcome events.
Table S4. Association of time‐updated potassium with outcomes adjusted for potential confounders including the time‐updated use of diuretics.
Table S5. Time‐updated subgroup analysis by four estimated glomerular filtration rate categories.
References
- 1. Savarese G, Xu H, Trevisan M, Dahlstrom U, Rossignol P, Pitt B, Lund LH, Carrero JJ. Incidence, predictors, and outcome associations of dyskalemia in heart failure with preserved, mid‐range, and reduced ejection fraction. JACC Heart Fail 2019;7:65–76. [DOI] [PubMed] [Google Scholar]
- 2. Desai AS, Liu J, Pfeffer MA, Claggett B, Fleg J, Lewis EF, McKinlay S, O'Meara E, Shah SJ, Sweitzer NK, Solomon S, Pitt B. Incident hyperkalemia, hypokalemia, and clinical outcomes during spironolactone treatment of heart failure with preserved ejection fraction: analysis of the TOPCAT trial. J Card Fail 2018;24:313–320. [DOI] [PubMed] [Google Scholar]
- 3. Nishihara T, Tokitsu T, Sueta D, Takae M, Oike F, Fujisue K, Usuku H, Takashio S, Hanatani S, Kanazawa H, Arima Y, Sakamoto K, Izumiya Y, Yamabe H, Kaikita K, Yamamoto E, Tsujita K. Serum potassium and cardiovascular events in heart failure with preserved left ventricular ejection fraction patients. Am J Hypertens 2018;31:1098–1105. [DOI] [PubMed] [Google Scholar]
- 4. Badr Eslam R, Öztürk B, Panzer S, Qin H, Duca F, Binder C, Rettl R, Dachs TM, Alasti F, Vila G, Bonderman D. Low serum potassium levels and diabetes – an unfavorable combination in patients with heart failure and preserved ejection fraction. Int J Cardiol 2020;317:121–127. [DOI] [PubMed] [Google Scholar]
- 5. Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, Bushinsky DA. Association of serum potassium with all‐cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol 2017;46:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palmer BF. Managing hyperkalemia caused by inhibitors of the renin‐angiotensin‐aldosterone system. N Engl J Med 2004;351:585–592. [DOI] [PubMed] [Google Scholar]
- 7. Ramírez E, Rossignoli T, Campos AJ, Muñoz R, Zegarra C, Tong H, Medrano N, Borobia AM, Carcas AJ, Frías J. Drug‐induced life‐threatening potassium disturbances detected by a pharmacovigilance program from laboratory signals. Eur J Clin Pharmacol 2013;69:97–110. [DOI] [PubMed] [Google Scholar]
- 8. Montford JR, Linas S. How dangerous is hyperkalemia? J Am Soc Nephrol 2017;28:3155–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol 2004;43:155–161. [DOI] [PubMed] [Google Scholar]
- 10. Zannad F, Ferreira JP, Pitt B. Potassium binders for the prevention of hyperkalaemia in heart failure patients: implementation issues and future developments. Eur Heart J Suppl 2019;21 (Suppl A):A55–A60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferreira JP, Rossello X, Pocock SJ, Rossignol P, Claggett BL, Rouleau JL, Solomon SD, Pitt B, Pfeffer MA, Zannad F. Spironolactone dose in heart failure with preserved ejection fraction: findings from TOPCAT. Eur J Heart Fail 2020;22:1615–1624. [DOI] [PubMed] [Google Scholar]
- 12. Ferreira JP, Dewan P, Jhund PS, Lorenzo‐Almorós A, Duarte K, Petrie MC, Carson PE, McKelvie R, Komajda M, Zile M, Zannad F, McMurray JJ. Covariate adjusted reanalysis of the I‐Preserve trial. Clin Res Cardiol 2020;109:1358–1365. [DOI] [PubMed] [Google Scholar]
- 13. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved trial. Lancet 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 14. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 15. Krogager ML, Torp‐Pedersen C, Mortensen RN, Kober L, Gislason G, Sogaard P, Aasbjerg K. Short‐term mortality risk of serum potassium levels in hypertension: a retrospective analysis of nationwide registry data. Eur Heart J 2017;38:104–112. [DOI] [PubMed] [Google Scholar]
- 16. Goyal A, Spertus JA, Gosch K, Venkitachalam L, Jones PG, Van den Berghe G, Kosiborod M. Serum potassium levels and mortality in acute myocardial infarction. JAMA 2012;307:157–164. [DOI] [PubMed] [Google Scholar]
- 17. Kovesdy CP, Matsushita K, Sang Y, Brunskill NJ, Carrero JJ, Chodick G, Hasegawa T, Heerspink HL, Hirayama A, Landman GW, Levin A, Nitsch D, Wheeler DC, Coresh J, Hallan SI, Shalev V, Grams ME; CKD Prognosis Consortium . Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta‐analysis. Eur Heart J 2018;39:1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kovesdy CP, Appel LJ, Grams ME, Gutekunst L, McCullough PA, Palmer BF, Pitt B, Sica DA, Townsend RR. Potassium homeostasis in health and disease: a scientific workshop cosponsored by the National Kidney Foundation and the American Society of Hypertension. J Am Soc Hypertens 2017;11:783–800. [DOI] [PubMed] [Google Scholar]
- 19. Solomon SD, McMurray JJ, Anand IS, Ge J, Lam CS, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin‐Colet J, Cleland J, Dungen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP; PARAGON‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 20. Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, Lam CS, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Shi VC, Lefkowitz MP, McMurray JJ. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON‐HF trial. JACC Heart Fail 2017;5:471–482. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 22. Harrell F. Regression Modeling Strategies: with Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001. [Google Scholar]
- 23. Ferreira JP. Abnormalities of potassium in heart failure. J Am Coll Cardiol 2020;75:2836–2850. [DOI] [PubMed] [Google Scholar]
- 24. Nunez J, Bayes‐Genis A, Zannad F, Rossignol P, Nunez E, Bodi V, Minana G, Santas E, Chorro FJ, Mollar A, Carratala A, Navarro J, Gorriz JL, Lupon J, Husser O, Metra M, Sanchis J. Long‐term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation 2018;137:1320–1330. [DOI] [PubMed] [Google Scholar]
- 25. Girerd N, Ferreira JP, Rossignol P, Zannad F. A tentative interpretation of the TOPCAT trial based on randomized evidence from the brain natriuretic peptide stratum analysis. Eur J Heart Fail 2016;18:1411–1414. [DOI] [PubMed] [Google Scholar]
- 26. Jering KS, Zannad F, Claggett B, Mc Causland FR, Ferreira JP, Desai A, Barkoudah E, McMurray JJ, Pfeffer MA, Solomon SD. Cardiovascular and renal outcomes of mineralocorticoid receptor antagonist use in PARAGON‐HF. JACC Heart Fail 2021;9:13–24. [DOI] [PubMed] [Google Scholar]
- 27. Wang HH, Hung CC, Hwang DY, Kuo MC, Chiu YW, Chang JM, Tsai JC, Hwang SJ, Seifter JL, Chen HC. Hypokalemia, its contributing factors and renal outcomes in patients with chronic kidney disease. PLoS One 2013;8:e67140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 29. Desai AS, Vardeny O, Claggett B, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Zile MR, Lefkowitz M, Shi V, Solomon SD. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril: a secondary analysis of the PARADIGM‐HF trial. JAMA Cardiol 2017;2:79–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Predictors of hypo‐ and hyperkalaemia throughout the follow‐up.
Table S2. Pairwise comparison for variables with significant differences in Table 1 .
Table S3. Landmark analysis assessing the impact of the number of hypo‐ and hyperkalaemia episodes and the average potassium levels during the first 8 months of the trial and subsequent primary outcome events.
Table S4. Association of time‐updated potassium with outcomes adjusted for potential confounders including the time‐updated use of diuretics.
Table S5. Time‐updated subgroup analysis by four estimated glomerular filtration rate categories.


