Abstract
Background
A proinflammatory diathesis, as measured by the neutrophil to lymphocyte ratio (NLR), heralds an adverse disease course for non–small cell lung cancer (NSCLC).
Methods
This post hoc analysis used data from the phase 3 OAK trial (NCT02008227), which randomized previously treated patients with NSCLC to atezolizumab or docetaxel. The main objective was assessing the differential impact of the pretreatment NLR on overall survival according to the treatment modality. In addition, patients' genomic characteristics were assessed according to their inflammatory status with a circulating free DNA (cfDNA) next‐generation sequencing (NGS) analysis.
Results
In all, 600 and 575 patients with NLR data were included in the atezolizumab and docetaxel cohorts, respectively, with a median NLR of 4 (interquartile range, 2.6–6.7) for the pooled population. An NLR ≥4 was associated with a positive smoking status (88.6% vs. 78.1%; p < .01), male sex (66.4% vs. 57.6%; p = .01), a worse performance status (71.3% vs. 55.2%; p < .01), a higher number of metastatic sites (63.2% vs. 51.6%; p = .01), squamous histology (32.1% vs. 21.4%; p < .01), and tissue KRAS mutations (30% vs. 18.7%; p = .02) but not with programmed death ligand 1 (PD‐L1) expression or the tissue epidermal growth factor receptor (EGFR)/anaplastic lymphoma kinase (ALK) status. A pretreatment NLR ≥4 was more strongly associated with mortality after atezolizumab (adjusted hazard ratio [HR], 1.64; 95% confidence interval [CI], 1.35–2.01) versus docetaxel (HR, 1.32; 95% CI, 1.08–1.60; multivariable [MVA] interaction p = .08). The HR for an increased risk of death for PD‐L1–negative/NLR ≥4 patients (compared with PD‐L1–positive/NLR <4 patients) was significantly higher in the atezolizumab cohort (MVA interaction p = .01). The exclusion of EGFR/ALK‐positive patients further increased the prognostic ability of the baseline NLR in favor of atezolizumab (MVA interaction p = .02). Pretreatment cfDNA data from NGS showed that patients with a high blood tumor mutation burden (cutoff, 16 mut/Mb) had a higher median NLR (4.6 vs. 3.7; p = .01). After adjustments for multiple comparisons, none of the selected variants of interest (EGFR, KRAS, TP53, KEAP1, STK11, SMARCA4, ARID1A, and targeted DNA damage response and repair genes) were significantly associated with the NLR.
Conclusions
A low baseline NLR identified patients with NSCLC who derived a greater survival benefit from atezolizumab in comparison with those identified in the docetaxel cohort. The NLR could complement PD‐L1 expression in tailoring treatment in this setting.
Keywords: atezolizumab, immune checkpoint inhibitors, immunotherapy, inflammation, lung cancer, neutrophil to lymphocyte ratio (NLR), non–small cell lung cancer (NSCLC)
Short abstract
The prognostic role of inflammatory indices in non–small cell lung cancer is already known, but an unanswered question exists about their possibly enhanced role with immunotherapy. In this post hoc analysis, the population of the OAK trial (NCT02008227) is used to demonstrate that the baseline neutrophil to lymphocyte ratio retains a differential effect depending on the treatment strategy (immunotherapy vs. chemotherapy).
1. INTRODUCTION
Responses to immunotherapy are underscored by a complex interplay between host and tumoral factors. In the context of metastatic non–small cell lung cancer (NSCLC), for which the treatment landscape is continuously evolving, additional predictive biomarkers beyond programmed death ligand 1 (PD‐L1) tumor expression are the focus of intense research efforts. 1 , 2
The presence of a systemic inflammatory reaction is deemed to reflect the release of cytokines by the tumor itself or as part of the host response against it. 3 , 4 Inflammation has been recognized for a long time as a pathogenic driver of cancer‐related cachexia and nutritional decline, features that portend an adverse prognosis for patients with NSCLC. 5 An excess of proinflammatory circulating cytokines often leads to a state of peripheral blood neutrophilia and reactive lymphopenia, which cause the neutrophil to lymphocyte ratio (NLR) to increase. 6 As a routinely available, reproducible, and inexpensive biomarker of activation of innate immunity, the NLR is one of the most investigated biomarkers in patients treated with immune checkpoint inhibitors (ICIs). 7
The pretreatment NLR has been investigated alone and in combination with platelet counts and serum lactate dehydrogenase to derive composite indices, which have proved to have a strong prognostic value for patients with NSCLC treated with ICIs across different treatment lines and PD‐L1 tumor expression levels. 8 , 9 , 10 However, for patients with lung cancer, the prognostic role of inflammatory indices is already known in other treatment settings and strategies, including surgery and chemotherapy 11 , 12 , 13 ; this leaves unanswered questions about their possibly enhanced role with immunotherapy.
The OAK trial (NCT02008227), a pivotal, randomized, phase 3 study, enrolled patients with advanced NSCLC to receive either atezolizumab or docetaxel, and it confirmed a survival benefit for immunotherapy over chemotherapy. Its sample size, primary end point, and eligibility criteria, which allowed patients with tumors of unselected histology/PD‐L1 expression levels to be enrolled, have made the OAK trial the optimal context for assessing the potentially different prognostic values of the NLR for chemotherapy and immunotherapy.
In light of expanding evidence showing that the NLR is associated with an immune‐exhausted tumor microenvironment, 14 we also sought to determine whether a high NLR was associated with the tumor mutation burden (TMB) or other genomic characteristics by using next‐generation sequencing (NGS) of pretreatment circulating free DNA (cfDNA) samples.
2. MATERIALS AND METHODS
The aim of this post hoc analysis was to evaluate the differential prognostic impact of systemic inflammation measured through the baseline NLR in patients with advanced NSCLC treated with either immunotherapy or chemotherapy. To this purpose, we performed this study with data from the phase 3 OAK trial 15 (NCT02008227), which included patients with measurable, previously treated NSCLC who had been randomly assigned to receive either atezolizumab or docetaxel. The study was conducted in accordance with the Declaration of Helsinki, and approval was obtained from all local ethics committees.
The study methodology has been published in detail previously; patients were randomized 1:1, and the primary end point was overall survival (OS). 15 , 16 , 17 Patients with oncogene‐addicted disease, including epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) receptor tyrosine kinase gene translocations, who had received prior targeted therapy were allowed to participate in the trial. 15 The OAK trial included 1225 patients in the secondary intention‐to‐treat population, but only patients who received the assigned treatment were included in this analysis. Efficacy data were extracted at the time of the secondary analysis; the data cutoff was January 23, 2017.
Pretreatment full blood count information to compute the baseline NLR for each patient was collected during the 28‐day screening window; patients with missing baseline NLR data were excluded from the analysis. For clinicopathologic and survival analyses, the NLR value was categorized as ≥4 or <4 in line with previous evidence from the literature. 8 , 18
We first evaluated the associations between the baseline NLR and clinicopathologic characteristics within the pooled population, and we subsequently reported the distribution of patients' features/baseline NLRs across the atezolizumab and docetaxel cohorts. Patients' OS was elected as the clinical end point of interest, whereas progression‐free survival (PFS) was set as a secondary end point. After the prognostic impact of the baseline NLR in the atezolizumab and docetaxel cohorts was described with univariable analyses, key clinicopathologic characteristics, including age (≥65 vs. <65 years), sex (male vs. female), Eastern Cooperative Oncology Group (ECOG) performance status (PS; −1 vs. 0), smoking status (ever vs. never smoker), tumor histology (squamous vs. nonsquamous), number of metastatic sites (>2 vs. ≤2), and number of prior therapies (1 vs. 2), were screened within the pooled population to build fixed multivariable models for OS and PFS and to evaluate the interaction term between the treatment modality (atezolizumab or docetaxel) and the NLR. We then adjusted the impact of the NLR in both cohorts by using the established fixed multivariable models.
In light of the continuous nature of the NLR, we additionally explored the impact of the NLR as a continuous covariate on the risk of death and disease progression/death, and we assessed its stratification ability for OS by using the top 20th, 40th, 60th, and 80th percentiles as tentative cutoffs in each cohort separately. 19
In order to evaluate the complementary effect of the NLR and PD‐L1 expression on patients' outcomes, tumor PD‐L1 was not included as an adjusting covariate in the fixed models and was assessed separately. We then stratified patients into three categories—good prognosis (NLR‐low and PD‐L1–positive status), intermediate prognosis (either NLR‐low status or PD‐L1–positive status), and poor prognosis (NLR‐high and PD‐L1–negative status)—as previously reported. 20 A centralized PD‐L1 assessment of tumor cells (TC1, TC2, or TC3) and tumor‐infiltrating immune cells (IC1, IC2, or IC3) was performed and previously described 15 , 21 ; for the purposes of this analysis, patients were categorized as positive or negative for PD‐L1 (a TC or IC score of at least 1 vs. no expression in either tumor cells or immune cells).
In light of the well‐established diminished efficacy of single‐agent PD‐1/PD‐L1 checkpoint inhibitors in patients with oncogene‐addicted tumors, 15 , 22 we performed an additional analysis of OS after the exclusion of patients whose tumors harbored EGFR mutations and ALK translocations.
2.1. Blood tumor mutation burden and targeted cfDNA analysis
For patients included in the primary analysis of the OAK trial (N = 850), peripheral blood was tested with the blood‐based FoundationOne Liquid CDx NGS assay, 23 which provided the blood tumor mutation burden (bTMB) and targeted cfDNA sequencing for 324 cancer‐related genes (details are available at https://assets.ctfassets.net/w98cd481qyp0/3a8jFw3KUjIU3RWPdcT9Ax/dcb2ffd6d8d9a40a65ccf663269cc39a/FoundationOne_Liquid_CDx_Label_Technical_Info.pdf).
Because in the pooled, retrospective analysis of the OAK and POPLAR trials a high bTMB identified patients who derived a significant improvement in their clinical outcomes, 23 we performed an additional analysis of baseline correlations among the NLR, bTMB, and targeted gene sequencing results. Because of their role in influencing clinical outcomes in patients with NSCLC treated with ICIs, seven genes of interest, including TP53, KRAS, EGFR, STK11, KEAP1, ARID1A, and SMARCA4, were selected for the analysis along with selected DNA damage response and repair genes from the panel defined for NSCLC by Ricciuti et al. 24 that were included in the FoundationOne Liquid CDx NGS assay (MLH1, MSH6, PMS2, ATM, ATR, CHEK1, CHEK2, BAP1, BARD1, BRCA1, BRCA2, BRIP1, PALB2, RAD51, RAD51C, RAD52, FANCA, FANCC, FANCG, FANCL, POLD1, POLE, ERCC4, and XRCC2).
We first reported the median baseline NLR across bTMB‐high patients (≥16 mut/Mb) and bTMB‐low patients (<16 mut/Mb) 23 and according to the mutational status for the key genes of interest. We then explored both the unadjusted and false discovery rate (FDR)–adjusted associations between the categorized NLR (≥4 vs. <4) and the mutational status of all the selected genes.
Patients with sample contamination >1%, a median exon coverage <800×, and a maximum somatic allele frequency (MSAF) < 1% were excluded from the bTMB analysis as previously done, 23 whereas those genetic variants with an MSAF <1% were considered to be wild type. Only variants that were pathogenetic or likely pathogenetic according to the manufacturer were considered for the analysis.
2.2. Statistical analysis
This post hoc analysis underwent a formal power calculation. The minimum sample size was estimated only for patients treated with immunotherapy; we hypothesized a 40% prevalence of patients with a baseline NLR ≥4 and assumed a possible survival benefit (a 40% reduction in the risk of death) for patients with a baseline NLR <4. With type I and type II error probabilities of 0.05 and 0.20, respectively, at least 148 death events were necessary, and at least 330 patients had to be included.
All pretreatment clinicopathologic features were reported with descriptive statistics as appropriate. OS and PFS were computed from the treatment start date to the date of death/last follow‐up and the date of disease progression/death, respectively. Patients not reported to have died at the time of the analysis were censored at the date of the last follow‐up, whereas patients without postbaseline information were censored at the randomization date plus 1 day as previously done. 15 Survival estimates were performed with the Kaplan–Meier method, were reported as medians with 95% confidence intervals (CIs), and were compared with the log‐rank test.
Cox regression was used for assessing the risk of death (OS) and disease progression (PFS), which was reported as hazard ratios (HRs) and 95% CIs. The two fixed models for multivariable analyses of OS and PFS were built with the pooled population via backward stepwise selection with enter/remove thresholds of p < .05 and p > .1, including covariates selected for their known prognostic role in NSCLC. After this, the impact of the NLR and the NLR/PD‐L1 combination was assessed in all the analyses with the established fixed multivariable models for OS and PFS, respectively. The α level for all the analyses was set to p < .05.
For the bTMB and targeted cfDNA exploratory analysis, the Kruskal–Wallis test was used for reporting the median baseline NLRs across the subgroups, whereas the FDR‐adjusted analysis was reported as a volcano plot with –log10(FDR q value) on the y‐axis and log2(odds ratio) on the x‐axis. Being a discovery setting, ≤0.1 was set as the threshold for statistical significance for q values. Statistical analyses were performed with MedCalc statistical software (version 18.11.3, 2019; MedCalc Software bvba, Ostend, Belgium [http://www.medcalc.org]). The volcano plot was designed with GraphPad Prism software (version 9.3.1 [471], December 2, 2021).
3. RESULTS
3.1. Patient characteristics
By the secondary analysis time data cutoff date, 1225 patients had been enrolled and randomized to receive treatment. Figure 1 presents the study flow diagram; after the exclusion of patients who did not receive the assigned treatment and did not have baseline NLR information, 600 patients treated with atezolizumab and 575 patients treated with docetaxel were included in the current analysis.
FIGURE 1.
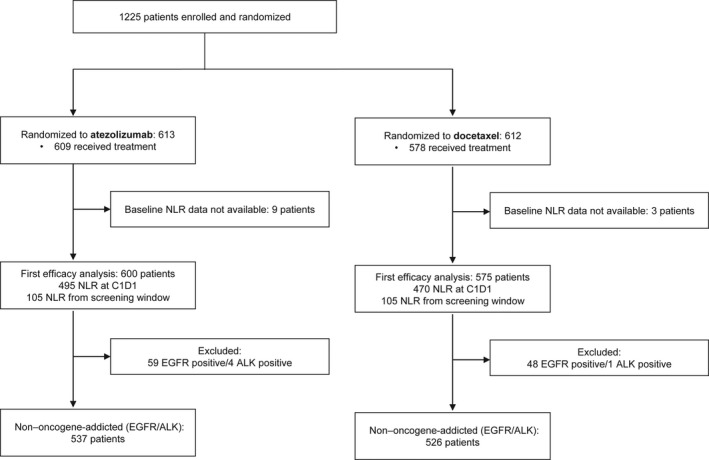
Study flow diagram. ALK indicates anaplastic lymphoma kinase; C1D1, cycle 1 day 1; EGFR, epidermal growth factor receptor; NLR, neutrophil to lymphocyte ratio
The NLR was computed from pretreatment full blood count information from cycle 1 day 1 for 495 patients (82.5%) and 470 patients (81.7%) in the atezolizumab and docetaxel cohorts, respectively. The median baseline NLR across the pooled population of 1175 patients was 4.0 (interquartile range [IQR], 2.6–6.7). Table 1 reports the baseline associations between clinicopathologic features and NLR categories (≥4 vs. <4); patients with a high NLR were more likely to be former/current smokers (88.6% vs. 78.1%; p < .0001), be male (66.4% vs. 57.6%; p = .0019), have an ECOG PS of 1 (71.3% vs. 55.2%; p < .0001), and have a higher number of metastatic sites (63.2% vs. 51.6%; p = .0001). In addition, their tumors were more likely to have a squamous histology (32.1% vs. 21.4%; p < .0001) and more likely to harbor KRAS mutations (30% vs. 18.7%), whereas no statistical trend toward a lower prevalence of EGFR‐positive tumors was reported in the NLR‐high group (8.9% vs. 12.7%; p = .0560). Table 2 summarizes patients' features according to the treatment received. The median baseline NLR was higher for the docetaxel cohort (4.3; IQR, 2.7–8.2) than the atezolizumab cohort (3.9; IQR, 2.5–5.8). However, the proportions of patients with a high NLR (≥4) were similar (53.2% vs. 48.2%; p = .0836), and no significant differences emerged between the two cohorts according to any of the included clinicopathologic characteristics.
TABLE 1.
Summary of the baseline associations between the clinicopathologic features of interest and the NLR categories (≥4 vs. <4)
| NLR <4 (n = 580), No. (%) | NLR ≥4 (n = 595), No. (%) | χ2 test p‐value | |
|---|---|---|---|
| PD‐L1 expression | |||
|
•TC3 or IC3 •TC2 or IC2 •TC2 or IC2 •Negative •Unknown |
41 (7.1) 104 (18.1) 170 (29.6) 260 (45.2) 5 |
56 (9.5) 96 (16.2) 173 (29.3) 266 (45.0) 4 |
.4726 |
| Age | |||
|
•≥65 years •<65 years |
263 (45.3) 317 (54.7) |
272 (45.7) 323 (54.3) |
.8989 |
| Smoking status | |||
|
•Former/current •Never |
453 (78.1) 127 (21.9) |
527 (88.6) 68 (11.4) |
<.0001 |
| Sex | |||
|
•Male •Female |
334 (57.6) 246 (42.4) |
395 (66.4) 200 (33.6) |
.0019 |
| ECOG PS | |||
|
•0 •1 |
260 (44.8) 320 (55.2) |
171 (28.7) 424 (71.3) |
<.0001 |
| Histological type | |||
|
•Squamous •Nonsquamous |
124 (21.4) 456 (78.6) |
191 (32.1) 404 (67.9) |
<.0001 |
| No. of metastatic sites | |||
|
•≤2 •>2 |
281 (48.4) 299 (51.6) |
219 (36.8) 376 (63.2) |
.0001 |
| No. of prior therapies | |||
|
•1 •2 |
452 (77.9) 128 (22.1) |
438 (73.6) 157 (26.4) |
.0844 |
| EGFR mutation | |||
|
•Negative •Positive •Unknown |
448 (87.3) 65 (12.7) 67 |
431 (91.1) 42 (8.9) 122 |
.0560 |
| EML4‐ALK translocation | |||
|
•Negative •Positive •Unknown |
306 (99.3) 2 (0.7) 272 |
264 (98.9) 3 (1.1) 328 |
.5416 |
| KRAS mutation | |||
|
•Negative •Positive •Unknown |
157 (81.3) 36 (18.7) 387 |
115 (70.9) 47 (29.1) 433 |
.0218 |
Abbreviations: ALK, anaplastic lymphoma kinase; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; IC, immune cells; NLR, neutrophil to lymphocyte ratio; PD‐L1, programmed death ligand 1; PS, performance status; TC, tumor cells.
TABLE 2.
Patient characteristics according to the received treatment
| Atezolizumab cohort (n = 600), No. (%) | Docetaxel cohort (n = 575), No. (%) | χ2 test p‐value | |
|---|---|---|---|
| Age | |||
|
•Median (range), years •≥65 years •<65 years |
63 (25–84) 271 (45.2) 329 (54.8) |
64 (34–85) 264 (45.9) 311 (54.1) |
.7974 |
| Smoking status | |||
|
•Former/current •Never |
493 (82.2) 107 (17.8) |
487 (84.7) 88 (15.3) |
.2443 |
| Sex | |||
|
•Male •Female |
372 (62.0) 228 (38.0) |
357 (62.1) 218 (37.9) |
.9755 |
| ECOG PS | |||
|
•0 •1 |
216 (36.0) 384 (64.0) |
215 (37.4) 360 (62.6) |
.6210 |
| Histological type | |||
|
•Squamous •Nonsquamous |
158 (26.3) 442 (73.7) |
157 (27.3) 418 (72.7) |
.7073 |
| No. of metastatic sites | |||
|
•≤2 •>2 |
270 (45.0) 330 (55.0) |
230 (40.0) 345 (60.0) |
.0833 |
| No. of prior therapies | |||
|
•1 •2 |
457 (76.2) 143 (23.8) |
433 (75.3) 142 (24.7) |
.7304 |
| PD‐L1 expression | |||
|
•Positive (TC1/TC2/TC3 and/or IC1/IC2/IC3) •Negative •Unknown |
340 (57.2) 254 (42.8) 6 |
255 (44.6) 317 (55.4) 3 |
.5313 |
| EGFR mutation | |||
|
•Negative •Positive •Unknown |
444 (74.0) 59 (9.8) 97 (16.2) |
435 (75.7) 48 (8.3) 92 (16.0) |
.6624 |
| EML4‐ALK translocation | |||
|
•Negative •Positive •Unknown |
306 (51.0) 4 (0.7) 290 (48.3) |
264 (45.9) 1 (0.2) 310 (53.9) |
.0808 |
| KRAS mutation | |||
|
•Negative •Positive •Unknown |
147 (24.5) 39 (6.5) 414 (69.0) |
125 (21.7) 44 (7.7) 406 (70.6) |
.4432 |
| NLR | |||
|
•Median (IQR) •≥4 •<4 |
3.9 (2.5–5.8) 289 (48.2) 311 (51.8) |
4.3 (2.7–8.2) 306 (53.2) 269 (46.8) |
.0836 |
Abbreviations: ALK, anaplastic lymphoma kinase; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; IC, immune cells; IQR, interquartile range; NLR, neutrophil to lymphocyte ratio; PD‐L1, programmed death ligand 1; PS, performance status; TC, tumor cells.
3.2. Prognostic effect of the NLR across the atezolizumab and docetaxel cohorts
The median follow‐up for the atezolizumab and docetaxel cohorts was 26.5 months (95% CI, 25.8–27.0 months) and 26.0 months (95% CI, 25.3–26.6 months), respectively. Among the atezolizumab recipients, patients with a high NLR achieved a median OS of 7.8 months (95% CI, 6.6–9.6 months; 217 events), whereas patients with a low NLR achieved a median OS of 17.1 months (95% CI, 15.2–20.0 months; 198 events) (HR, 1.88; 95% CI, 1.55–2.28; p < .0001; Figure 2A). Among the docetaxel recipients, patients with a high NLR achieved the same median OS of 7.8 months (95% CI, 6.8–9.1 months; 242 events), whereas patients with a low NLR achieved a median OS of 12.5 months (95% CI, 10.8–13.8 months; 189 events) (HR, 1.49; 95% CI, 1.23–1.81; p < .0001; Figure 2B). Table S1 reports the multivariable model for the pooled population with the selected covariates for OS (ECOG PS, histology, and number of metastatic sites) and PFS (ECOG PS, smoking status, histology, number of metastatic sites, and number of prior therapies).
FIGURE 2.
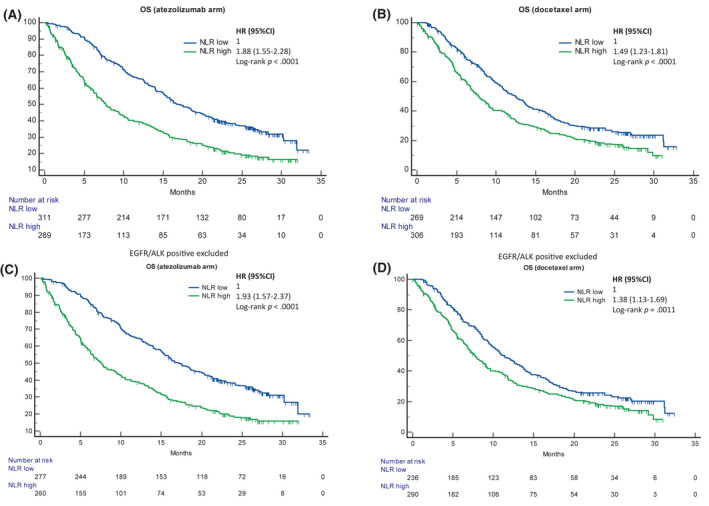
Kaplan–Meier survival estimates for OS according to the NLR. (A) Atezolizumab cohort including oncogene‐addicted patients: NLR low (17.1 months; 95% CI, 15.2–20.0 months; 198 events) versus NLR high (7.8 months; 95% CI, 6.6–9.6 months; 217 events) (p < .0001; HR, 1.88; 95% CI, 1.55–2.28). (B) Docetaxel cohort including oncogene‐addicted patients: NLR low (12.5 months; 95% CI, 10.8–13.8 months; 189 events) versus NLR high (7.8 months; 95% CI, 6.8–9.1 months; 242 events) (p < .0001; HR, 1.49; 95% CI, 1.23–1.81). (C) Atezolizumab cohort excluding EGFR/ALK‐positive patients: NLR low (17.1 months; 95% CI, 15.1–20.0 months; 178 events) versus NLR high (7.6 months; 95% CI, 6.2–9.6 months; 199 events) (p < .0001; HR, 1.93; 95% CI, 1.57–2.37). (D) Docetaxel cohort excluding EGFR/ALK‐positive patients: NLR low (11.3 months; 95% CI, 9.7–13.3 months; 175 events) versus NLR high (7.7 months; 95% CI, 6.8–8.9 months; 230 events) (p = .0011; HR, 1.38; 95% CI, 1.13–1.69). ALKindicates anaplastic lymphoma kinase; CI, confidence interval; EGFR, epidermal growth factor receptor; HR, hazard ratio; NLR, neutrophil to lymphocyte ratio; OS, overall survival
After adjustments for the selected variables, the HR for an increased risk of death for patients with a high NLR was numerically higher in the atezolizumab cohort (HR, 1.64; 95% CI, 1.35–2.01) versus the docetaxel cohort (HR, 1.32; 95% CI, 1.08–1.60) with a multivariable interaction p value of .0869 (Figure 3A).
FIGURE 3.
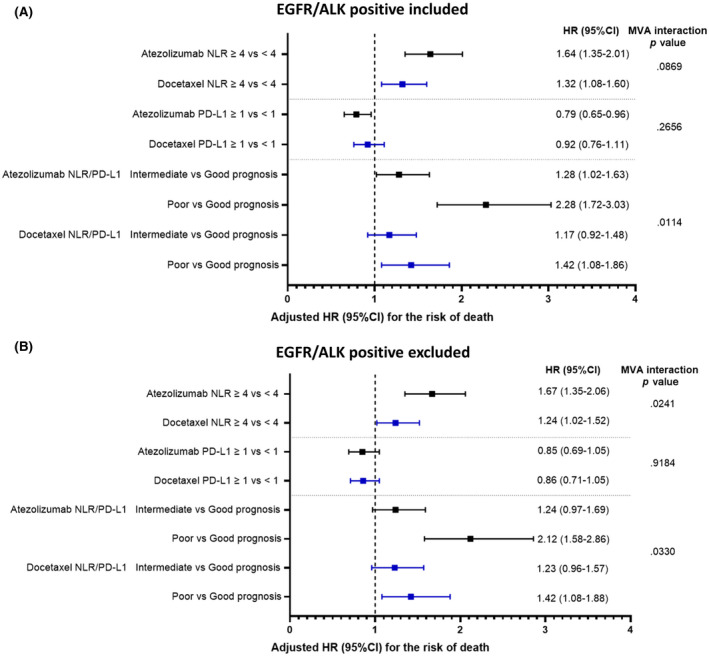
Forest plot graphs reporting the adjusted HRs for the risk of death across the two cohorts according to the NLR, PD‐L1 expression, and NLR/PD‐L1 complementation. (A) Whole population (including EGFR/ALK‐positive patients). (B) Population after the exclusion of EGFR/ALK‐positive patients. Adjusting factors included the Eastern Cooperative Oncology Group performance status (1 vs. 0), histology (squamous vs. nonsquamous), and number of metastatic sites (≤2 vs. >2). The interaction terms through the same MVA model included the pooled population. ALK indicates anaplastic lymphoma kinase; CI, confidence interval; EGFR, epidermal growth factor receptor; HR, hazard ratio; MVA, multivariable; NLR, neutrophil to lymphocyte ratio; PD‐L1, programmed death ligand 1
Even when it was used as continuous covariate, the risk of death with increasing NLR was numerically higher in the atezolizumab cohort (HR, 1.08; 95% CI, 1.06–1.10) versus the docetaxel cohort (HR, 1.02; 95% CI, 1.01–1.02). Similarly, the additional analysis using the top 20th, 40th, 60th, and 80th percentiles as exploratory cutoffs confirmed the higher prognostication of the NLR in the atezolizumab cohort; this was supported by the correspondingly higher HRs for an increased risk of death for patients with a high NLR with each level in comparison with the docetaxel cohort (Figure S1).
3.3. Differential complementary role of NLR and PD‐L1 expression
As reported in Table 1, we did not find any association between tumor/immune cell PD‐L1 expression and the baseline NLR categories (p = .4726), and this suggested their independence. We therefore evaluated their possible complementary role in defining patients' prognoses across the two cohorts. The combination of PD‐L1 and NLR was significantly associated with the median OS in both the atezolizumab cohort (p < .0001) and the docetaxel cohort (p = .0010; Figure S2A,B), although the HRs for an increased risk of death for patients with a poor prognosis versus patients with good prognostic factors were higher for the atezolizumab recipients.
After adjustments for the selected variables, the HR for an increased risk of death for patients with a poor prognosis (PD‐L1–negative with a high NLR vs. PD‐L1–positive with a low NLR) was significantly higher in the atezolizumab cohort (HR, 2.28; 95% CI, 1.72–3.03) versus the docetaxel cohort (HR, 1.42; 95% CI, 1.08–1.86) with a multivariable interaction p value of 0.0114 (Figure 3A).
3.4. Differential prognostic effect of the NLR in patients with non–oncogene‐addicted NSCLC
After the exclusion of 59 and 48 patients with EGFR/ALK‐positive tumours, 537 patients (89.5%) and 526 patients (91.5%) were included in the atezolizumab and docetaxel cohorts, respectively (Figure 1). Patients with a high NLR achieved a shorter median OS in both the atezolizumab cohort (HR, 1.93; 95% CI, 1.57–2.37; log‐rank p < .0001) and the docetaxel cohort (HR, 1.38; 95% CI, 1.13–1.69; log‐rank p = .0011; Figure 2C,D). After adjustments with the fixed multivariable model, the HR for an increased risk of death for patients with a high NLR was significantly higher in the atezolizumab cohort (HR, 1.67; 95% CI, 1.35–2.06) versus the docetaxel cohort (HR, 1.24; 95% CI, 1.02–1.52) with a multivariable interaction p value of 0.0241 (Figure 3B). Figure S2C,D reports the Kaplan–Meier estimates of OS according to the PD‐L1/NLR complementation across the two cohorts after the exclusion of patients with EGFR/ALK‐positive tumours, and it confirms the differential prognostic effect between immunotherapy and chemotherapy. Similarly, the multivariable HR for an increased risk of death for patients with two factors (PD‐L1 negativity and high NLR vs. PD‐L1 negativity and low NLR) was significantly higher in the atezolizumab cohort (HR, 2.12; 95% CI, 1.58–2.86) versus the docetaxel cohort (HR, 1.42; 95% CI, 1.08–1.88) with a multivariable interaction p value of .0330 (Figure 3B).
3.5. PFS analysis
In the overall population, patients with a high NLR achieved shorter PFS in comparison with patients with a low NLR in both the atezolizumab cohort (HR, 1.33; 95% CI, 1.13–1.57; log‐rank p = .0007; Figure S3A) and the docetaxel cohort (HR, 1.31; 95% CI, 1.10–1.55; log‐rank p = .0017; Figure S3B). Similar results were found when we compared the impact on PFS of the PD‐L1/NLR combination, which significantly affected both the atezolizumab cohort (log‐rank p = .0001) and the docetaxel cohort (log‐rank p = .0317; Figure S3C,D).
The multivariable HR for an increased risk of disease progression/death for patients with a high NLR was statistically significant in the atezolizumab cohort (HR, 1.34; 95% CI, 1.13–1.60) but was not statistically significant in the docetaxel cohort (HR, 1.19; 95% CI, 0.99–1.42) with a multivariable interaction p value of .3687. The multivariable HR for an increased risk of disease progression/death for patients with two factors (PD‐L1 negativity and high NLR vs. PD‐L1 negativity and low NLR) was significant in the atezolizumab cohort (HR, 1.60; 95% CI, 1.25–2.05) but was not significant in the docetaxel cohort (HR, 1.09; 95% CI, 0.85–1.39) with a significant multivariable interaction p value of .0257 (Figure S4).
Even when it was used as a continuous covariate, the risk of disease progression/death with increasing NLR was numerically higher in the atezolizumab cohort (HR, 1.05; 95% CI, 1.03–1.06) versus the docetaxel cohort (HR, 1.01; 95% CI, 1.00–1.02).
3.6. bTMB and targeted cfDNA exploratory analysis
After the exclusion of ineligible patients due to sample contamination, exon coverage, and MSAF, 636 patients (54.1%) and 749 patients (63.7%) were included in the bTMB and targeted cfDNA exploratory analyses, respectively. The median baseline NLR of patients with a low bTMB (3.7; IQR, 2.5–6.1) was significantly lower than that of patients with a high bTMB (4.6; IQR, 2.9–8.3; p = .0120; Figure 4A). Figure S5A–H reports the distribution of median NLRs according to the selected gene of interest; with the exception of KRAS mutations (mutant, 5.1; IQR, 2.8–8.4; wild type, 3.8; IQR, 2.5–6.2; p = .0367) and STK11 mutations (mutant, 5.3; IQR, 3.0–8.9; wild type, 3.8; IQR, 2.5–6.3; p = .0339), we did not find any other significant associations. The volcano plot presented in Figure 4B summarizes the FDR‐adjusted analysis including all 15 genes of interest, which confirmed that none of the included mutations were independently associated with the NLR status.
FIGURE 4.
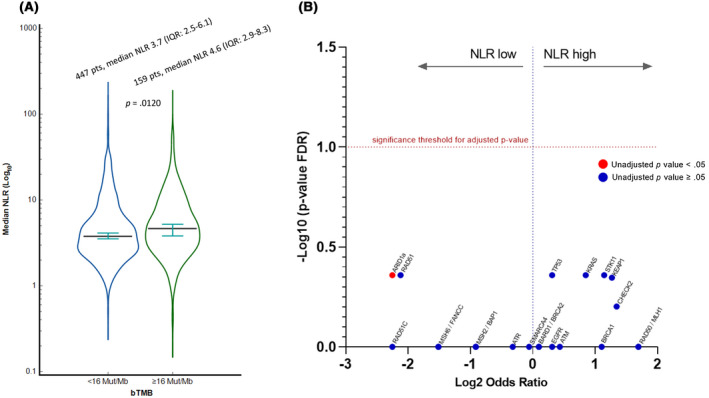
(A) Violin plot reporting the median baseline NLR (log10) according to the categorized bTMB (≥16 vs. <16 mut/Mb). (B) Volcano plot summarizing the FDR‐adjusted targeted cfDNA analysis. The only gene mutation significantly associated with the NLR (NLR low) was ARID1A(unadjusted p value < .05). After adjustments for multiple comparisons, none of the 15 selected genes of interest were associated with the NLR. bTMB indicates blood tumor mutation burden; cfDNA, circulating free DNA; FDR, false discovery rate; IQR, interquartile range; NLR, neutrophil to lymphocyte ratio; pts, patients
4. DISCUSSION
Tumor‐promoting inflammation is a recognized hallmark of cancer, 25 and the systemic effect of inflammation can be easily and reproducibly measured in the clinic with inexpensive prognostic indices such as the NLR. 26 Patients with an increased NLR display the hallmark of exhausted T‐cell immunity, 6 which lends credence to the NLR as a putative stratifying biomarker for an oncological indication with a rich therapeutic landscape where ICIs have been integrated and replaced standard chemotherapy.
This is the first study to demonstrate in a prospectively accrued cohort of patients randomized to receive cytotoxic therapy or ICI therapy that a high pretreatment NLR could identify a group of patients with a numerically identical median OS of 7.8 months regardless of whether they received docetaxel or atezolizumab. On the other hand, immunotherapy was associated with enhanced benefits in those with a low NLR, for whom the median OS was significantly extended to 17.1 months versus 12.5 months with docetaxel. Although patients with a high NLR experienced worse outcomes in both the atezolizumab and docetaxel cohorts, the HR for an increased risk of death demonstrated a stronger effect in the atezolizumab group than the docetaxel group in all analyses.
The OAK study was commenced in March 2014, and the protocol allowed the enrollment of patients with EGFR/ALK‐positive tumours, a subgroup that would be excluded subsequently from PD‐1/PD‐L1 monotherapy studies on account of the intrinsic immune refractoriness of these tumors. 22 It is then not surprising to see that our findings have been further magnified in the analysis excluding patients with EGFR/ALK‐addicted tumours, where the significant interaction p value confirmed the differential prognostic ability of the NLR between immunotherapy and chemotherapy.
The preferential effect on OS observed in atezolizumab‐treated patients has strong mechanistic foundations. Neutrophils are in fact effector cells involved in the innate and adaptive immune responses. Although neutrophilia is a common clinical feature of many patients with cancer, neutrophils are also an important part of the immune infiltrate in different types of cancer as tumor‐associated neutrophils. 27 The dual role of both circulating and tumor‐associated neutrophils has long been debated, as they have been reported to have direct cytotoxic effects and to be able to inhibit antitumoral cytotoxic T cells, secreting proinflammatory cytokines and growth factors, including interleukin 6 (IL‐6), IL‐8, IL‐12, metalloproteinases, and TGF‐β, and promoting the angiogenetic switch. 6 , 27 , 28 On the other hand, lymphocytes are immune cells with cytolytic activity that are known for their ability to limit tumorigenesis and tumor progression. Both circulating and tumor‐infiltrating lymphocytes have been reported to correlate with immune responses and enhanced efficacy of ICIs across a wide variety of cancers, 29 , 30 whereas in NSCLC, differential expression of immune checkpoint molecules in the tumor microenvironment, including tumor‐infiltrating lymphocytes, has been linked to both survival and relapse. 31 Conversely, prolonged lymphocytopenia, with a reduction of CD4+ and CD8+ T circulating cells, could be a direct result of cancer‐related systemic inflammation and may affect the ability to sustain an effective anticancer immune response. 3 , 32
We made an interesting observation based on the NLR/PD‐L1 combination analysis. There is an absence of a baseline association between the NLR and PD‐L1 expression levels, and this suggests a possible nonoverlapping role for these biomarkers. In the OAK study population, the beneficial effect of atezolizumab over docetaxel was similar across the PD‐L1 TC/IC categories. 15 This is also reflected in the current study, where we did not observe any differential effect or significant interactions according to the PD‐L1 expression (≥1% vs. negative) between the atezolizumab and docetaxel cohorts. However, when we combined the NLR and the PD‐L1 status into three categories, the differential prognostic ability between immunotherapy and chemotherapy was further magnified; this was confirmed by the significant interaction p value reported in the analyses with and without patients with EGFR/ALK‐positive tumours. More specifically, patients with a high NLR and PD‐L1–negative tumors seemed to be the driver of that difference: They achieved more pronounced detrimental outcomes in comparison with patients with a low NLR and PD‐L1–positive tumors with immunotherapy versus chemotherapy.
The PFS analysis can be viewed from the same perspective. The increased risk of disease progression for patients with a high NLR was statistically significant among immunotherapy recipients only and was slightly higher than that observed with docetaxel, whereas the combination of a low NLR and PD‐L1 positivity produced a trend similar to that observed for OS. In addition, the significant interaction p value confirmed the differential diagnostic effect for the risk of disease progression according to the treatment modality, and this suggests a possible predictive role of the NLR/PD‐L1 integration for immunotherapy.
However, our results cannot be interpreted without consideration of the baseline associations between the NLR and clinicopathologic characteristics. A high NLR was significantly associated with clinical features that could underly a deranged proinflammatory status, including male sex, 33 a higher ECOG PS, 34 squamous histology, 35 and a higher disease burden, 36 which are also hallmarks of worse outcomes overall. The significant association with the smoking status 37 could also explain the relationship between a high NLR and KRAS mutations, as these two features have already been linked in several studies. 38 , 39
The bTMB and targeted cfDNA analysis provided some further insights. Interestingly, we found that patients with a high bTMB had a significantly higher median NLR than patients with a low bTMB. Although the TMB–NLR correlation with their possibly complementary roles has already been reported, 19 this is the first study highlighting a link between the proinflammatory systemic response and cfDNA genomic features. Notably, the bTMB–NLR association could also be explained by the NLR–smoking relationship 40 and by the association between the NLR and the number of metastatic sites, as cfDNA shed into the bloodstream might be related to metastatic spread, 41 and TMB can be related to disease burden in NSCLC. 42 The targeted cfDNA analysis showed that a higher median NLR was reported also for patients with a KRAS mutation and those with an STK11 mutation; however, no significant association with the NLR categorical status was confirmed with the FDR‐adjusted analysis.
Exploring the potentially different prognostic abilities of inflammatory indices according to the treatment modality has become even more relevant with the advent of chemoimmunotherapy combinations, which have become viable first‐line options for the majority of patients, regardless of PD‐L1 expression. 43 The current results suggest that the baseline NLR could complement PD‐L1 expression in stratifying patients who might be candidates to receive an ICI‐based therapy. In the debate about the best first‐line approach for patients with high PD‐L1 expression, between single‐agent ICIs and chemoimmunotherapy combinations, a conservative approach could be considered for some patients with a low NLR, who derive a greater benefit from immunotherapy than chemotherapy when we consider patients' comorbidities and disease burden. Randomized trials basing stratification on predefined biomarker‐specific efficacy end points will be crucial for tailoring the right therapies for patients.
Our study has several limitations, which are mainly due to the fact that it is an exploratory, retrospective analysis of subgroups that were not prespecified. Although validated in the first‐line setting, 8 , 20 the cutoff of 4 for the NLR is not widely recognized or used for NSCLC. 44 However, the median value for the included population was exactly 4, and this allowed us to assume a good level of reliability for our analysis. An additional limit to the reproducibility of our results lies in the PD‐L1 evaluation, as the current routine evaluation is based on the tumor proportion score, which measures tumor cells only, whereas the OAK trial included a combined score accounting for both tumor and tumor‐infiltrating cells. 15 , 21 Although patients on baseline systemic steroids (greater than or equal to the equivalent of 10 mg of prednisone) were not permitted in the trial, we have to consider the mandatory steroid premedication for docetaxel, which might have caused the slightly higher median NLR in the chemotherapy cohort.
Despite the aforementioned limitations, our study is the first one providing evidence of a differential prognostic ability of the NLR between immunotherapy and chemotherapy in NSCLC from a randomized clinical trial population. The NLR proved to be an effective tool for identifying patients with a deranged proinflammatory response, who are less likely to respond to ICIs. In particular, patients with a low NLR derived a greater benefit from immunotherapy in comparison with chemotherapy. In addition, the baseline NLR and PD‐L1 expression could be combined to improve patient selection for PFS and OS, whereas the differential PFS stratification also suggests a possible predictive role for NLR/PD‐L1 complementation in this setting. Further validation through independent, prospective cohorts is warranted before our findings are considered for the decision‐making process in clinical practice.
AUTHOR CONTRIBUTIONS
Alessio Cortellini: Conceptualization, methodology, software, formal analysis, investigation, resources, visualization, project administration, validation, writing–original draft, and writing–review and editing. Biagio Ricciuti: Methodology, validation, and writing–review and editing. Hossein Borghaei: Validation and writing–review and editing. Abdul Rafeh Naqash: Validation and writing–review and editing. Antonio D'Alessio: Validation and writing–review and editing. Claudia A. M. Fulgenzi: Validation and writing–review and editing. Alfredo Addeo: Conceptualization, validation, and writing–review and editing. Giuseppe L. Banna: Conceptualization, validation, and writing–review and editing. David James Pinato: Supervision, validation, writing–original draft, and writing–review and editing. All authors contributed to the publication according to the International Committee of Medical Journal Editors guidelines for authorship (study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision), and all authors read and approved the submitted version of the manuscript (and any substantially modified version involving their contributions to the study). Each author has agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even parts in which the author was not personally involved, are appropriately investigated and resolved, and the resolution is documented in the literature.
CONFLICT OF INTEREST
Alessio Cortellini reports speaker and consulting fees from AstraZeneca, MSD, BMS, Roche, Novartis, and Eisai. Hossein Borghaei reports institutional grants from BMS, Lilly, and Amgen; consulting fees from BMS, Lilly, Genentech, Pfizer, Merck, EMD‐Serono, Boehringer Ingelheim, AstraZeneca, Novartis, Genmab, Regeneron, BioNTech, Amgen, Axiom, PharmaMar, Takeda, Mirati, Daiichi, Guardant, Natera, Oncocyte, BeiGene, iTEO, Jazz, Janssen, and Da Volterra; participation on boards for Novartis, Takeda, and Incyte; stock/stock options in Sonnet Bio, Inspirna, and Nuleai; and the receipt of equipment, materials, drugs, medical writing, gifts, or other services from Amgen and BMS. Antonio D'Alessio has declared travel support from Roche. Alfredo Addeo reports consulting fees from Amgen, AstraZeneca, Roche, Astellas, Takeda, BMS, MSD, Pfizer, Merck, and Novartis and speaker fees from Amgen and Novartis. Giuseppe L. Banna reports payments or honoraria from AstraZeneca and Astellas Pharma. David James Pinato reports lecture fees from ViiV Healthcare and Bayer HealthCare; payments or honoraria from BMS, Roche, Eisai, and the Falk Foundation; travel expenses from BMS and Bayer HealthCare; consulting fees from Mina Therapeutics, Eisai, Roche, AstraZeneca, Da Volterra, and BMS; and research funding (to his institution) from MSD and BMS. The other authors made no disclosures.
Supporting information
Appendix XXX
ACKNOWLEDGMENTS
This publication is based on research using data from Roche that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication. Biagio Ricciuti would like to acknowledge the Society of Immunotherapy of Cancer‐Astrazeneca for the support provided with the Young Investigator Award. David J Pinato would like to acknowledge the support provided by Wellcome Trust Strategic Fund (PS3416), the Associazione Italiana per la Ricerca sul Cancro (AIRC MFAG Grant ID 25697), the NIHR Imperial Biomedical Research Centre (BRC), the Imperial Experimental Cancer Medicine Centre (ECMC) and the Imperial College Tissue Bank. Antonio D’Alessio would like to acknowledge the European Association for the Study of the Liver for the support provided with the Andrew Burroughs Fellowship. Alessio Cortellini would like to acknowledges the support provided by the NIHR Imperial BRC.
The last two authors contributed equally to this article.
DATA AVAILABILITY STATEMENT
The data sets used for this study are available on formal request from Vivli, Inc.
REFERENCES
- 1. Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non–small cell lung cancer (NSCLC). Cancer. 2020;15(126):260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Camidge DR, Doebele RC, Kerr KM. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat Rev Clin Oncol. 2019;16(6):341–355. [DOI] [PubMed] [Google Scholar]
- 3. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer‐related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. [DOI] [PubMed] [Google Scholar]
- 4. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Op den Kamp CM, Langen RC, Minnaard R, et al. Pre‐cachexia in patients with stages I–III non–small cell lung cancer: systemic inflammation and functional impairment without activation of skeletal muscle ubiquitin proteasome system. Lung Cancer. 2012;76(1):112–117. [DOI] [PubMed] [Google Scholar]
- 6. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431–446. [DOI] [PubMed] [Google Scholar]
- 7. Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil‐to‐lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta‐analysis. Onco Targets Ther. 2018;11:955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Banna GL, Cortellini A, Cortinovis DL, et al. The lung immuno‐oncology prognostic score (LIPS‐3): a prognostic classification of patients receiving first‐line pembrolizumab for PD‐L1 >/= 50% advanced non–small‐cell lung cancer. ESMO Open. 2021;6(2):100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diem S, Schmid S, Krapf M, et al. Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) as prognostic markers in patients with non–small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. [DOI] [PubMed] [Google Scholar]
- 10. Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non–small cell lung cancer. JAMA Oncol. 2018;4(3):351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non–small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peng B, Wang YH, Liu YM, Ma LX. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non–small cell lung cancer: a systemic review and meta‐analysis. Int J Clin Exp Med. 2015;8(3):3098–3106. [PMC free article] [PubMed] [Google Scholar]
- 13. Yao Y, Yuan D, Liu H, Gu X, Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non–small cell lung cancer patients treated with first‐line platinum‐based chemotherapy. Cancer Immunol Immunother. 2013;62(3):471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alessi JV, Ricciuti B, Alden SL, et al. Low peripheral blood derived neutrophil‐to‐lymphocyte ratio (dNLR) is associated with increased tumor T‐cell infiltration and favorable outcomes to first‐line pembrolizumab in non–small cell lung cancer. J Immunother Cancer. 2021;9(11):e003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non–small‐cell lung cancer (OAK): a phase 3, open‐label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fehrenbacher L, von Pawel J, Park K, et al. Updated efficacy analysis including secondary population results for OAK: a randomized phase III study of atezolizumab versus docetaxel in patients with previously treated advanced non–small cell lung cancer. J Thorac Oncol. 2018;13(8):1156–1170. [DOI] [PubMed] [Google Scholar]
- 17. Mazieres J, Rittmeyer A, Gadgeel S, et al. Atezolizumab versus docetaxel in pretreated patients with NSCLC: final results from the randomized phase 2 POPLAR and phase 3 OAK clinical trials. J Thorac Oncol. 2021;16(1):140–150. [DOI] [PubMed] [Google Scholar]
- 18. Banna GL, Cortellini A, Cortinovis DL, et al. Corrigendum to ‘The lung immuno‐oncology prognostic score (LIPS‐3): a prognostic classification of patients receiving first‐line pembrolizumab for PD‐L1 ≥ 50% advanced non–small‐cell lung cancer’: [ESMO Open volume 6, issue 2, April 2021, 100078]. ESMO Open. 2021;6(3):100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valero C, Lee M, Hoen D, et al. Pretreatment neutrophil‐to‐lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun. 2021;12(1):729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Banna GL, Signorelli D, Metro G, et al. Neutrophil‐to‐lymphocyte ratio in combination with PD‐L1 or lactate dehydrogenase as biomarkers for high PD‐L1 non–small cell lung cancer treated with first‐line pembrolizumab. Transl Lung Cancer Res. 2020;9(4):1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non–small‐cell lung cancer (POPLAR): a multicentre, open‐label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. [DOI] [PubMed] [Google Scholar]
- 22. Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR‐mutated non–small cell lung cancer—a meta‐analysis. J Thorac Oncol. 2017;12(2):403–407. [DOI] [PubMed] [Google Scholar]
- 23. Gandara DR, Paul SM, Kowanetz M, et al. Blood‐based tumor mutational burden as a predictor of clinical benefit in non–small‐cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24(9):1441–1448. [DOI] [PubMed] [Google Scholar]
- 24. Ricciuti B, Recondo G, Spurr LF, et al. Impact of DNA damage response and repair (DDR) gene mutations on efficacy of PD‐(L)1 immune checkpoint inhibition in non–small cell lung cancer. Clin Cancer Res. 2020;26(15):4135–4142. [DOI] [PubMed] [Google Scholar]
- 25. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 26. Leach M. Interpretation of the full blood count in systemic disease—a guide for the physician. J R Coll Physicians Edinb. 2014;44(1):36–41. [DOI] [PubMed] [Google Scholar]
- 27. Shaul ME, Fridlender ZG. Tumour‐associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019;16(10):601–620. [DOI] [PubMed] [Google Scholar]
- 28. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blessin NC, Spriestersbach P, Li W, et al. Prevalence of CD8(+) cytotoxic lymphocytes in human neoplasms. Cell Oncol (Dordr). 2020;43(3):421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lotzova E. Role of human circulating and tumor‐infiltrating lymphocytes in cancer defense and treatment. Nat Immun Cell Growth Regul. 1990;9(4):253–264. [PubMed] [Google Scholar]
- 31. Guo H, Diao L, Zhou X, et al. Artificial intelligence–based analysis for immunohistochemistry staining of immune checkpoints to predict resected non–small cell lung cancer survival and relapse. Transl Lung Cancer Res. 2021;10(6):2452–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ostroumov D, Fekete‐Drimusz N, Saborowski M, Kuhnel F, Woller N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci. 2018;75(4):689–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Di Florio DN, Sin J, Coronado MJ, Atwal PS, Fairweather D. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. 2020;31:101482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dolan RD, Laird BJA, Klepstad P, et al. An exploratory study examining the relationship between performance status and systemic inflammation frameworks and cytokine profiles in patients with advanced cancer. Medicine (Baltimore). 2019;98(37):e17019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bozinovski S, Vlahos R, Anthony D, et al. COPD and squamous cell lung cancer: aberrant inflammation and immunity is the common link. Br J Pharmacol. 2016;173(4):635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dall’Olio FG, Marabelle A, Caramella C, et al. Tumour burden and efficacy of immune‐checkpoint inhibitors. Nat Rev Clin Oncol. 2022;19(2):75–90. [DOI] [PubMed] [Google Scholar]
- 37. Colak Y, Afzal S, Lange P, Nordestgaard BG. Smoking, systemic inflammation, and airflow limitation: a Mendelian randomization analysis of 98 085 individuals from the general population. Nicotine Tob Res. 2019;21(8):1036–1044. [DOI] [PubMed] [Google Scholar]
- 38. Chapman AM, Sun KY, Ruestow P, Cowan DM, Madl AK. Lung cancer mutation profile of EGFR, ALK, and KRAS: meta‐analysis and comparison of never and ever smokers. Lung Cancer. 2016;102:122–134. [DOI] [PubMed] [Google Scholar]
- 39. Ferrer I, Zugazagoitia J, Herbertz S, John W, Paz‐Ares L, Schmid‐Bindert G. KRAS‐mutant non–small cell lung cancer: from biology to therapy. Lung Cancer. 2018;124:53–64. [DOI] [PubMed] [Google Scholar]
- 40. Yoshida K, Gowers KHC, Lee‐Six H, et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature. 2020;578(7794):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oxnard GR, Paweletz CP, Sholl LM. Genomic analysis of plasma cell‐free DNA in patients with cancer. JAMA Oncol. 2017;3(6):740–741. [DOI] [PubMed] [Google Scholar]
- 42. Stein MK, Pandey M, Xiu J, et al. Tumor mutational burden is site specific in non–small‐cell lung cancer and is highest in lung adenocarcinoma brain metastases. JCO Precis Oncol. 2019;3:1–13. [DOI] [PubMed] [Google Scholar]
- 43. Rocco D, Della Gravara L, Battiloro C, Gridelli C. The role of combination chemo‐immunotherapy in advanced non–small cell lung cancer. Expert Rev Anticancer Ther. 2019;19(7):561–568. [DOI] [PubMed] [Google Scholar]
- 44. Jiang T, Bai Y, Zhou F, et al. Clinical value of neutrophil‐to‐lymphocyte ratio in patients with non–small‐cell lung cancer treated with PD‐1/PD‐L1 inhibitors. Lung Cancer. 2019;130:76–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix XXX
Data Availability Statement
The data sets used for this study are available on formal request from Vivli, Inc.


