Summary
Poales are one of the most species‐rich, ecologically and economically important orders of plants and often characterise open habitats, enabled by unique suites of traits. We test six hypotheses regarding the evolution and assembly of Poales in open and closed habitats throughout the world, and examine whether diversification patterns demonstrate parallel evolution.
We sampled 42% of Poales species and obtained taxonomic and biogeographic data from the World Checklist of Vascular Plants database, which was combined with open/closed habitat data scored by taxonomic experts. A dated supertree of Poales was constructed. We integrated spatial phylogenetics with regionalisation analyses, historical biogeography and ancestral state estimations.
Diversification in Poales and assembly of open and closed habitats result from dynamic evolutionary processes that vary across lineages, time and space, most prominently in tropical and southern latitudes. Our results reveal parallel and recurrent patterns of habitat and trait transitions in the species‐rich families Poaceae and Cyperaceae. Smaller families display unique and often divergent evolutionary trajectories.
The Poales have achieved global dominance via parallel evolution in open habitats, with notable, spatially and phylogenetically restricted divergences into strictly closed habitats.
Keywords: biogeography, evolution, evolutionary transitions, grasslands, grass‐like plants, savannas, spatial phylogenetics
Introduction
Open and closed habitats are distinct categories of terrestrial environments (Bond, 2019), each with unique ecology and differing in ground layer vegetation strongly constrained by available light (Ratnam et al., 2011; Bond, 2022). Open habitats, comprising nearly 60% of land area (Dinerstein et al., 2017), include treetops that provide suitable habitats for epiphytes, areas too extreme to support trees (e.g. deserts, high altitudes, tundra and rock walls), and grasslands – regions that could potentially support trees but are dominated by grass‐like plants because of disturbances (Strömberg, 2011; Buisson et al., 2022). Grasslands occupy a wide biogeographic range and cover nearly 40% of the terrestrial biosphere, including tropical and subtropical savannas, boreal and temperate prairies, and Eurasian steppes (Bond, 2019; Buisson et al., 2022). They provide habitat for a wide diversity of animals and plants, and support the livelihood of over 1 billion people worldwide (Buisson et al., 2022). Until now, biogeographical and evolutionary studies of open habitats have focussed on regional scales (e.g. Solofondranohatra et al., 2020) or on the most diverse family occupying these habitats, Poaceae (e.g. Edwards et al., 2010; Linder et al., 2018; Gallaher et al., 2022), although other closely related families can be equally diverse and dominant components of their respective open habitats (Linder & Rudall, 2005; Barrett, 2013).
The ability of plants to occur in either open or closed habitat has evolved repeatedly across the tree of life, in some cases possibly through parallel evolution – the repeated evolution of similar traits in closely related lineages (Bailey et al., 2017; Durán‐Castillo et al., 2022) – or through divergent evolution involving large evolutionary distances between populations (or species) in genotypic or phenotypic space (Bolnick et al., 2018). As the morphological and physiological traits of plants are linked to their habitat preferences, the repetitive evolution of traits suggests parallel adaptive evolution (Bolnick et al., 2018). To identify macroevolutionary processes, such as whether evolution is parallel or divergent, it is important to determine the appropriate phylogenetic scope to avoid excluding key lineages that unveil important patterns (Folk et al., 2018). Large comparative studies including comprehensive sampling across a single higher level lineage present opportunity to distinguish which traits might be exceptional at smaller compared with larger phylogenetic scales (e.g. genus vs order; Beaulieu & O'Meara, 2018).
Globally dominant in open habitats, Poales are among the most species‐rich orders of angiosperms, comprising 14 families and 24 302 species (APG IV, 2016; Govaerts, 2022; Fig. 1; Table 1). Poales families differ in their species richness and distribution, yet those exhibiting high species richness tend to be cosmopolitan and exhibiting variation in life form and ecological traits (Ricklefs & Renner, 1994). The two largest families, Poaceae and Cyperaceae, are among the three largest monocot families and 10 largest plant families (Govaerts, 2022) and both have a cosmopolitan distribution. Whereas mid‐sized families show latitudinal bias (e.g. Restionaceae in austral temperate regions; Bromeliaceae in the American tropics) or are nearly restricted to a single continent (e.g. Rapateaceae in South America). Those that are species‐poor (e.g. Typhaceae, Ecdeiocoleaceae) tend to be geographically and/or ecologically restricted. Most families in Poales are largely limited to open habitats or are able to occupy both open and closed habitats. Most are also capable of long‐distance dispersal by wind, animals and water (Linder et al., 2018; Martín‐Bravo et al., 2019; Vera‐Paz et al., 2023). While all Poales families are found in tropical areas, only select clades have diversified extensively in temperate areas (e.g. Poaceae subfamily Pooideae, Carex, Juncaceae; Vigeland et al., 2013; Martín‐Bravo et al., 2019; Ambroise et al., 2020; Schubert et al., 2020). These lineages tend to be widespread throughout the northern hemisphere, with many genera occupying full Holarctic distributions despite their contingent species displaying strong partitioning of geographic and niche space (Spalink et al., 2016, 2019).
Fig. 1.

Representatives of 12 Poales families. (a) Tillandsia tovarensis (Bromeliaceae), epiphytic in cloud forest, Kuelap, N Peru. (b) Insect‐pollinated Rhynchospora colorata (Cyperaceae), in forest gaps, S Ecuador. (c) Ecdeiocolea rigens (Ecdeiocoleaceae), arid heath, SW Australia. (d) Paepalanthus ensifolius (Eriocaulaceae), in cloud forest, Podocarpus National Park, S Ecuador. (e) Flagellaria indica (Flagellariaceae), rocky savannah, NW Australia. (f) Mayaca fluviatilis (Mayacaceae), wetlands, Singapore. (g) Micraira sp. Purnululu (Poaceae), a rapid‐resurrection species from sandstone pavements in NW Australia. (h) Guacamaya superba (Rapataceae) in a sedge and grass swamp in E Colombia. (i) Lepidobolus preissii (Restionaceae) from sandy heath, SW Australia. (j) Prionium serratum (Thurniaceae) in fynbos, Cape Province, South Africa. (k) Sparganium japonicum (Typhaceae) from wetlands in E Russia. (l) Xyris complanata (Xyridaceae) from an ephemeral wetland in savannah, NW Australia. Photos by Russell Barrett; except for (f, h, j, k), all posted on iNaturalist as CC‐BY‐NC; (f) by CheongWeei Gan; (h) by Carlos Eduardo; (j) by Linda Hibbin; (k) by Sergei Prokopenko.
Table 1.
Summary of number of species, ancestral habitat and phyloregions corresponding to crown nodes for Poales.
| Family | Total | Studied | Studied % | Closed | Phyloregion | Habitat |
|---|---|---|---|---|---|---|
| Poaceae | 11 994 | 5341 | 45 | 449 | Afrotropical | Closed |
| Cyperaceae | 5870 | 2690 | 46 | 398 | Neotropic | Open |
| Bromeliaceae | 3564 | 1066 | 30 | 551 | Neotropic | Open |
| Eriocaulaceae | 1206 | 348 | 29 | 6 | Neotropic | Open |
| Restionaceae | 552 | 453 | 82 | 3 | Austral | Open |
| Juncaceae | 514 | 209 | 41 | 5 | Palearctic | Open |
| Xyridaceae | 406 | 13 | 3 | 1 | Neotropic | Open |
| Rapateaceae | 97 | 24 | 25 | 2 | Neotropic | Open |
| Typhaceae | 74 | 26 | 35 | 0 | Palearctic | Open |
| Thurniaceae | 8 | 4 | 50 | 0 | Neotropic | Open |
| Flagellariaceae | 5 | 4 | 80 | 2 | Indomalayan | Closed |
| Mayacaceae | 5 | 1 | 20 | 0 | Neotropic | Open |
| Joinvilleaceae | 4 | 2 | 50 | 2 | Indomalayan | Closed |
| Ecdeiocoleaceae | 3 | 2 | 67 | 0 | Austral | Open |
| Total | 24 302 | 10 183 | 1419 | |||
| % | 42 | 14 |
We expect large‐scale comparative studies of Poales to reveal that diversification both in and out of open habitats in multiple families occurred repeatedly and in parallel around the world. The ancestor of Poales is hypothesised to have occupied open, and seasonally dry, nutrient‐poor, Gondwanan habitats during the Cretaceous (Givnish et al., 2000; Linder & Rudall, 2005; Bouchenak‐Khelladi et al., 2014) and would have been herbaceous, with typical monocot features including rhizomes, monocarpic tillers, C3 photosynthesis and wind pollination (Dahlgren et al., 1985). Several lineages of Poales have entered closed habitats since the Late Cretaceous, but such lineages are predominantly species‐poor (e.g. Flagellariaceae), except for Bromeliaceae which has rapidly diversified during the Miocene (Givnish et al., 2011, 2014). Conversely, several species‐rich Poales lineages have diversified in open habitats, apparently in conjunction with Crassulacean Acid Metabolism (CAM) and C4 photosynthetic systems (Bouchenak‐Khelladi et al., 2014). This generalisation, however, is based on incomplete sampling (below 2% of species; Bouchenak‐Khelladi et al., 2014), which is likely to obscure underlying patterns and processes, and thus remains to be tested with more comprehensive sampling.
Coupling the most inclusive phylogeny of Poales produced to date with comprehensive geographic sampling and habitat scoring, here we integrate historical biogeography, ancestral state estimation and spatial phylogenetics. With this, we test hypotheses regarding the evolution and assembly of Poales in open and closed habitats throughout the world, analyse the impact of evolutionary history on phylogenetic regionalisation and examine whether diversification patterns demonstrate parallel or divergent evolution. Spatial phylogenetic analyses (Mishler et al., 2014) are increasingly used to evaluate the geographic structure of lineage assembly (Holt et al., 2013; Thornhill et al., 2016; Zhang et al., 2022), to link evolutionary processes to the manifestation of biodiversity through time and space (Carter et al., 2022; Nitta et al., 2022; Sanbonmatsu & Spalink, 2022), and for the development of conservation policies (Sechrest et al., 2002; Gonzalez‐Orozco et al., 2016; Zhang et al., 2021). While many spatial phylogenetic metrics exist, emerging methods can identify hotspots of remarkable phylogenetic diversity and distinguish between areas of significant palaeo‐ and neo‐endemism (Mishler et al., 2014). Such analyses provide insight into the spatial patterns of evolutionary processes, integrating phylogenetic information (i.e. branch lengths and species relationships) with the geographic ranges of species and clades. When coupled with functional traits, this inclusion of evolutionary information provides a more complete understanding of macroecological processes and phylogenetic patterns of regionalisation (Tucker et al., 2017; Spalink et al., 2018). To our knowledge, this is the first study to incorporate modern spatial phylogenetic approaches with historical biogeographical analyses of a plant order on a global scale. We capitalise on this data set to link our understanding of how lineages evolve with how they assemble at both deep and more shallow phylogenetic scales, avoiding the edge effects of regionally restricted analyses (e.g. Spalink et al., 2018) while minimising bias stemming from incomplete sampling.
We test the following hypotheses: (H1) Phylogenetic regionalisation will reflect a tropical origin and extensive temperate diversification only of select clades of Poales. (H2) Given that relatively few clades have diversified in temperate and boreal habitats, and given the propensity of long‐distance dispersal in Poales, we expect that north‐ and south‐temperate regions will be more phylogenetically similar to each other than either is to tropical regions. (H3) Evolutionary transitions between open and closed habitats have occurred frequently, rapidly, and in parallel in Poales, as three of the largest families (Poaceae, Cyperaceae, and Bromeliaceae) have diversified in both habitats. (H4) Evolutionary transitions to, and diversification within, open habitats have occurred in synchrony in different parts of the world and across lineages, with the rapid accumulation of species beginning in the Eocene as open habitats became more widespread. We expect our increased sampling over previous studies to reveal that diversification in open habitats did not begin with the now‐dominant Poaceae, but rather with multiple families simultaneously around the world. (H5) These historical processes have resulted in high phylogenetic diversity and endemism across the tropics, where most Poalean families have a hypothesised origin, and low endemism in northern temperate habitats, where lineages are widespread even though individual species are spatially restricted. (H6) Across Poales, centres of neo‐endemism will occur on young islands and mountainous regions, whereas palaeo‐endemism will occur in areas with high bioclimatic stability since the Eocene. We expect no significant neo‐ or palaeo‐endemism in the temperate north, either in Poales as a whole or in individual families, given that these regions are recently assembled and composed of predominantly widespread lineages.
Materials and Methods
Phylogenetic reconstruction
We produced a family‐level phylogenomic backbone using 353 nuclear loci (Angiosperms353; Johnson et al., 2019). The sampling for the backbone aimed towards 50% of accepted genera and included newly generated data. Details on data production, sequence assembly and species tree construction are presented in Supporting Information Notes S1 and in Baker et al. (2022). Briefly, we inferred the phylogenomic backbone using a multi‐species coalescent framework (MSC). Gene trees were inferred using IQ‐Tree 2 (Minh et al., 2020). TreeShrink (Mai & Mirarab, 2018) was used to identify outliers that significantly increased tree space. Alignment and tree building was repeated for those genes with outlier trees. All gene trees were trimmed for poorly supported branches (UFBS < 30%) before use in the MSC analysis in ASTRAL‐III (Zhang et al., 2018). To obtain a species tree with branch lengths proportional to the genetic distance, we ranked the genes according to the congruence of their resulting trees to the species tree using SortaDate (Smith et al., 2018) and then concatenated the alignments of the 25 most congruent genes. Using the MSC species tree as topological constraint and this concatenated alignment, a new phylogram was inferred in IQ‐Tree 2. For more details on library preparation and data analyses, please refer to Baker et al. (2022).
We inferred separate trees for five groups of families identified with the backbone phylogeny: (1) Bromeliaceae + Typhaceae; (2) Rapateaceae + Thurniaceae + Juncaceae + Cyperaceae + Mayacaceae (the cyperid clade); (3) Xyridaceae + Eriocaulaceae (the xyrid clade); (4) Restionaceae; and (5) Joinvilleaceae + Flagellariaceae + Ecdeiocoleaceae + Poaceae. Available sequences (Table S1) were retrieved from GenBank (Benson et al., 2010). We downloaded all available sequences per marker while disregarding those corresponding to hybrids and not determined to species level, with the exception of the cyperid clade where we preferentially used sequences included in the phylogenetic reconstruction of Elliott et al. (2022). Nomenclatural reconciliation was conducted using the R package TAXIZE (Chamberlain & Szocs, 2013) and ‘World Checklist of Vascular Plants’ (WCVP; Govaerts, 2022). We selected one sequence per marker per taxon. Sequence matrices for the five clades were aligned using Mafft v.7.453 (Katoh & Standley, 2013), edited manually in AliView v.1.26 (Larsson, 2014) and concatenated. Trees were then inferred under a GTRCAT model in RAxML v.8.2.12 (Stamatakis, 2014), using rapid bootstrapping with 100 replicates followed by a thorough maximum likelihood search on the Czech National Grid Infrastructure. The preliminary phylogenetic trees were manually checked for obviously spurious species placements. Corrected matrices were realigned with outgroup taxa (Table S1). Phylogenetic reconstructions were then conducted using constraint trees derived from the backbone tree.
For time‐calibration, we used a mix of primary and secondary calibrations to match dates of major clades to the best‐available evidence from previous studies focussing on monocots. The backbone and clade trees were time‐calibrated using penalised likelihood as implemented in treePL, with the smoothing value identified through cross‐validation (Smith & O'Meara, 2012). We used secondary calibration points for the backbone tree, setting fixed ages for the family crown nodes (Table S2) obtained from the plastome tree of Givnish et al. (2018). Separate analyses were conducted for Poaceae and Cyperaceae, incorporating additional fossil and secondary priors within the clades using best available information (Spalink et al., 2016; Martín‐Bravo et al., 2019; Gallaher et al., 2022; see Table S2). For the remaining clade trees, the root ages were set to one. All subtrees were then grafted into the backbone tree after scaling their age to that of the corresponding nodes in the dated backbone tree.
Species distributions and habitats
We used WCVP to create a species presence/absence matrix in each botanical country (Level 3) as specified by the International Working Group on Taxonomic Databases for Plant Sciences (TDWG; Brummitt et al., 2001). Species missing in the phylogeny were removed from the matrix. Occurrence in open, closed, or both habitats was scored from the literature, especially regional floras, in addition to taxonomic expertise. Habitats noted as ‘forests’ in floristic treatments, interpreted as closed canopy at least during the active growing season, were scored as ‘closed’. We scored species occurring in open and closed habitats as ‘both’. For Bromeliaceae, species occurring on sun‐exposed rock‐walls or treetops were scored as ‘open’. A full list of taxa included in the study and their habitat is available in Table S3.
Regionalisation
To determine phylogenetic patterns of regionalisation in Poales, we first calculated phylogenetic beta‐diversity across all botanical countries. We then used three metrics to identify the optimal number of phyloregions: K‐means and silhouette scores (Hartigan & Wong, 1979; Rousseeuw, 1987), the elbow method (Salvador & Chan, 2004; Zhao et al., 2011) and the gap statistic (Tibshirani et al., 2001). For each analysis, we set the maximum number of phyloregions to 20, allowing for the possibility of high levels of regionalisation. The elbow and K‐means approach each identified 13 phyloregions as optimal, whereas the gap statistic identified 20 phyloregions. For simplicity, we used 13 phyloregions for downstream comparative analyses, with regions partitioned to the optimal number identified above using non‐metric multidimensional scaling (NMDS) and hierarchical dendrogram clustering based on a beta‐diversity similarity matrix of botanical countries. To determine nestedness of the 13 regions, we completed a final analysis that limited the number of phyloregions to three. Regionalisation analyses were conducted using the R packages phyloregion v.1.0.6 (Daru et al., 2017, 2020a,b), cluster v.2.1.3 (Maechler et al., 2022) and stats v.4.1.2 (R Core Team, 2021). To summarise the phylogeny and visualise spatial and temporal patterns of lineage assembly in these phyloregions, we used the R package phytools v.1.2.0 (Revell, 2012) to construct nonparametric lineage through time plots for the open and closed habitat species of each family. This summary approach does not account for extinction or missing data, but was selected over a model‐based estimates of λ (birth rate) and μ (death rate), given that these are difficult to estimate from trees containing only extant species (Louca & Pennell, 2020). We do not use these plots to infer diversification rates, but rather to visualise the approximate timing of assembly of each family in each phyloregion given the phylogeny.
Ancestral state estimations
For ancestral area estimation, we expected that the dispersal–extinction–cladogenesis (DEC) model would have the most appropriate parameters for geographic scale and biology of our study system (Ree et al., 2005; Ree & Smith, 2008; see Notes S2). For comparison to DEC, we also ran the DIVA‐like model. While we acknowledge the criticisms of the additional J parameter (Ree & Sanmartín, 2018), we ran DEC and DIVA with and without J given that Poales has a propensity for long‐distance dispersal that could lead to speciation. We selected the best model among the four using AICw scores. We used stochastic mapping to estimate the number of dispersal events between phyloregions. Models, model testing and stochastic mapping were implemented using the R package BioGeoBEARS v1.1.2 (Matzke, 2013, 2014). The geographical unit of comparison was the Poales phyloregions identified above, but simplified to 7 to reduce the state‐space explored by the model (see inset map in Fig. 4 later). We did not implement the BAYAREA‐like model, which allows ‘widespread sympatric speciation’, a parameter that is not biologically plausible given the biology of Poales and the geographic scope of our study system. This parameter allows a cosmopolitan species to sympatrically give rise to two other cosmopolitan species, resulting in estimations favouring large ancestral areas and multiple widespread sympatric speciation events.
Fig. 4.
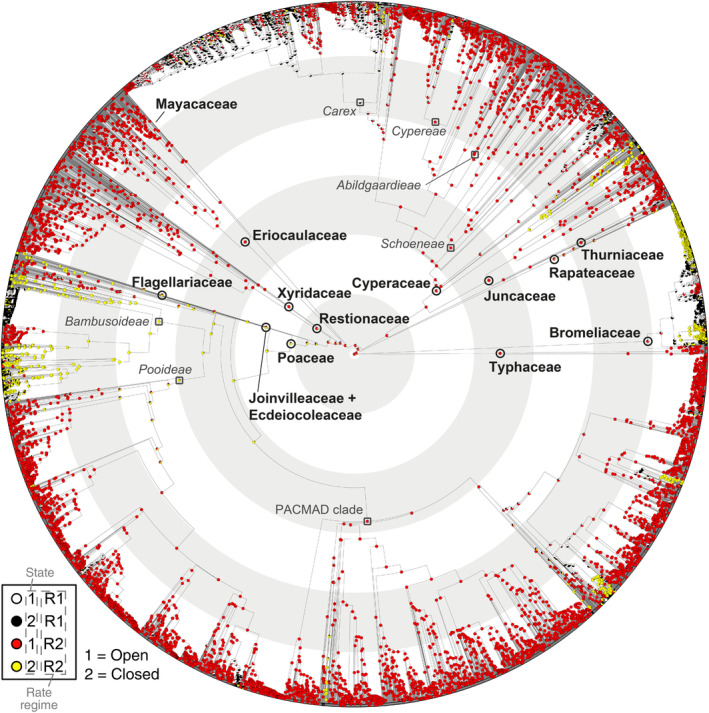
Ancestral states of open/closed habitats based on Generalized Hidden Markov models, with hidden rates model with two categories and asymmetric rates, implemented with the corHMM package in R. The crown nodes of the families within Poales are shown with black circles, whereas dark grey squares are used to depict lineages important for the study's interpretations. Concentric light grey/white rings underlying the phylogeny indicate time slots of 20 Myr intervals. Detailed transition rates between the states and rate are given in Table S5.
To estimate ancestral states of open/closed habitat and the transition rates between them, we used Generalised Hidden Markov models, as implemented in the function corHMM in the R package corHMM v.2.8 (Boyko & Beaulieu, 2021). We ran ‘symmetric rates’ and ‘all rates differ’ Markov models separately with a hidden states model with two categories and with a standard model without hidden rates (one category of rates) fitted during the analyses. Akaike information criterion (AIC) was used to select the best fitting model, and ancestral states in the phylogeny were inferred with maximum likelihood and stochastic mapping. We then linked the output of the best‐fitting corHMM models to the DEC analysis to summarise spatial patterns of open/closed habitat transitions, identifying the nodes at which character states shifted, and the geographical areas that those ancestors were inferred to have occupied based on the DEC analysis.
Spatial phylogenetics
We used the R packages Picante v.1.8.2 (Kembel et al., 2010) and canaper v.0.0.2 (Nitta & Iwasaki, 2021) to calculate phylogenetic diversity (PD; Faith, 1992) and phylogenetic endemism (PE; Rosauer et al., 2009), as well as to perform categorical analysis of neo‐ and palaeo‐endemism (CANAPE; Mishler et al., 2014) across botanical countries. To calculate the standardised effect size of PD and PE, we used two different sequential randomisation algorithms – swap and curveball. The swap algorithm shuffles species presence/absence across the community matrix, while maintaining species ranges sizes and species richness within communities (Gotelli & Entsminger, 2003). The curveball algorithm identifies species occurring in only one of two randomly selected communities, then distributes those species across communities while maintaining richness in communities (Strona et al., 2014). For both randomisations, we performed 1000 replications of 100 000 iterations each. These were followed by two‐tailed tests to determine whether PD and PE were significantly over‐dispersed or clustered. CANAPE methods follow those outlined in Mishler et al. (2014) and Nitta & Iwasaki (2021). For PD, PE and CANAPE, analyses were conducted for all Poales combined, as well as separately for the six largest families (Bromeliaceae, Cyperaceae, Eriocaulaceae, Juncaceae, Poaceae and Restionaceae). In addition, PD was calculated for both open and closed habitat species.
Results
Our final phylogeny encompassed 10 100 species (41.6% of Poales; Table 1). All families and 88% of genera in the order were represented. The percentage of species sampled varied from highest in Restionaceae (82%, 453 species), medium for the largest families Poaceae (45%, 5341 species) and Cyperaceae (46%, 2690 species), to lowest in Xyridaceae (3%, 13 species). Habitat scoring was completed for > 80% of species (Table S3). Only 14% of species occur in closed habitats. Ages of major clades were consistent with the previous studies on which the calibrations were based.
Regionalisation
Phylogenetic regionalisation identified 13 regions (Fig. 2a) nested into three major zones (Fig. S1): (I) Temperate (1: temperate North America + Russian far east; 5: central and eastern North America; 2: northern Africa [excluding Algeria] + Arabian peninsula [excluding Yemen] + southwestern Asia; 6: Europe + mediterranean Eurasia + Algeria; 8: northeastern Asia; 12: central and eastern China; 13: north central Pacific); 4: Patagonia + Antarctica + western and southern Australia + New Zealand; (II) American tropics (3: Central‐ + South America [excluding central and eastern Brazil + Patagonia]; 11: central and eastern Brazil) and (III) Palaeotropics (7: Sub‐Saharan Africa [excluding central and western Africa; Madagascar + Mozambique] + Yemen + Mauritania; 9: Indomalayan, comprising India + southeastern Asia + northeastern Australia + Madagascar + Mozambique; 10: central and western tropical Africa [excluding Mauritania]). A NMDS plot shows these 13 regions separated latitudinally, falling into three groups, where northern and southern hemisphere temperate regions form a single cluster separated from the tropics, with the latter separated into Neotropics (excluding Patagonia) and Palaeotropics (Figs 2a, S1).
Fig. 2.
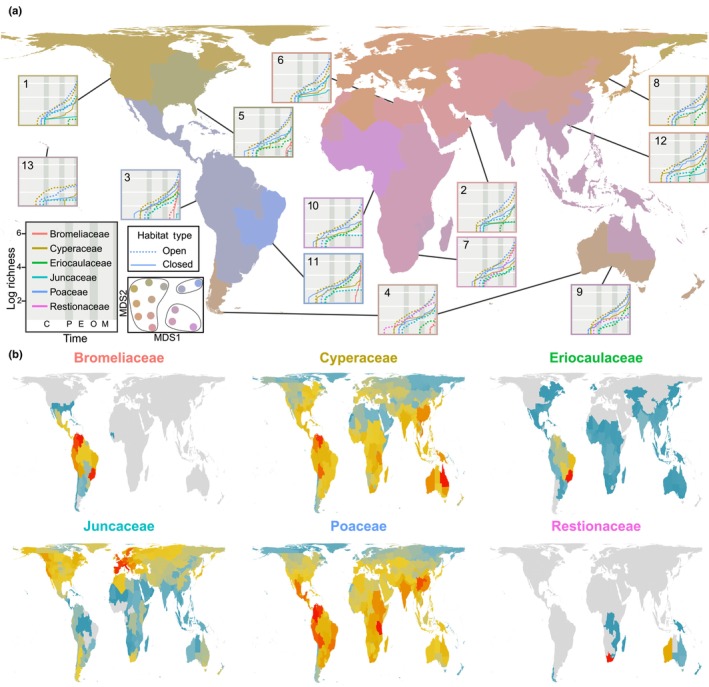
Global phylogenetic patterns of regionalisation in Poales. ( a) Phylogenetic regionalisation of the six largest families of Poales. Graphs represent the logarithmic species richness of the six families through time within each of the 13 phyloregions identified using the elbow and K‐means approach, where dotted lines represent open habitat lineages and solid lines indicate closed habitat lineages. A geological timeline is located in the inset in the far left‐hand corner, with the following abbreviations: Cretaceous (C: 66–56 Myr); Paleocene (P: 66–56 Myr); Eocene (E: 56–33.9 Myr); Oligocene (O: 33.9–23 Myr) and Miocene (M: 23–5.3 Myr). Inset to the right of the geological timeline shows nestedness of the 13 regions based on non‐metric multidimensional scaling (NMDS), where each colour dot represent a phyloregion and the three clusters each with similar colour dots represent the three major botanical kingdoms (Temperate, Neotropics, Palaeotropics). (b) Phylogenetic diversity (PD) of the six most speciose families of Poales, with red and dark blue indicating botanical countries with relatively high and low PD values, respectively. Botanical regions indicated by grey in (b) lack species for the respective family.
Families differ in their stem age and lineage accumulation over time among the 13 regions (Fig. 2a). Considering the six largest families, Poaceae or Cyperaceae are the oldest in all regions, except for the Restionaceae which are oldest in temperate Australia. Bromeliaceae is the youngest family. Smaller families (Eriocaulaceae and Juncaceae) are younger than Poaceae and Cyperaceae in regions where they occur. Poaceae and Cyperaceae occur in all regions, Juncaceae is nearly cosmopolitan but is poorly represented in tropical regions, and the other nine families are restricted to one or few regions (Fig. 2a,b).
In all tropical regions, Poales – and specifically Poaceae – diversified first in closed habitats before lineages began accumulating in open habitats (Fig. 2a). By contrast, in northern temperate regions open habitat lineages – specifically, Cyperaceae – diversified first (Fig. 2a). The exception to this latter pattern is in the eastern Nearctic, where closed habitat Poaceae were the first to diversify. However, this exception is likely driven by the inclusion of some subtropical Caribbean islands and southern Florida in this phyloregion. The initial diversification of Poaceae in most temperate regions included both open and closed habitat lineages. Likewise, but in tropical regions only, the initial diversification of open and closed habitat Cyperaceae was concurrent. Open habitat Poaceae displayed the greatest accumulation of lineages (especially during the Oligocene and Miocene) followed by open habitat Cyperaceae in all but the north central Pacific phyloregion, where there was a big lapse in time until Cyperaceae accumulated in closed habitats (Fig. 2a). In all phyloregions, open habitat species richness is higher than closed habitat species richness in all families except Bromeliaceae.
Ancestral state estimations
The BioGeoBEARS model with the highest AICw was the DEC model followed by DEC + J and DIVA models (Table S4; Fig. S1). Subsequent analyses are based on the DEC model (Fig. 3). Poales are inferred to have originated in the Neotropics in Western Gondwana (current South America; Fig. 3a). The crown nodes of most families occur in the Cretaceous (e.g. Cyperaceae, Eriocaulaceae, Juncaceae, Poaceae and Restionaceae) to the Neogene (e.g. Bromeliaceae; Fig. 3a). The family crown nodes are distributed in all major zones (Fig. 3a; Table 1): Bromeliaceae, Cyperaceae, Eriocaulaceae, Rapataceae and Xyridaceae are assigned a Neotropic origin, while Flagellariaceae and Poaceae are placed in the Palaeotropics. The Holarctic region is ancestral for the Juncaceae and Typhaceae, whereas the Austral region is ancestral for the Restionaceae. Below family rank, Schoeneae originated in the Austral region, Carex and Pooideae in Eurasia, Cypereae and the PACMAD clade in Sub‐Saharan Africa, Abildgaardieae in Indomalayan region (including NE Australia, Mozambique, and Madagascar) and Bambusoideae in the Neotropics (Fig. 3a).
Fig. 3.
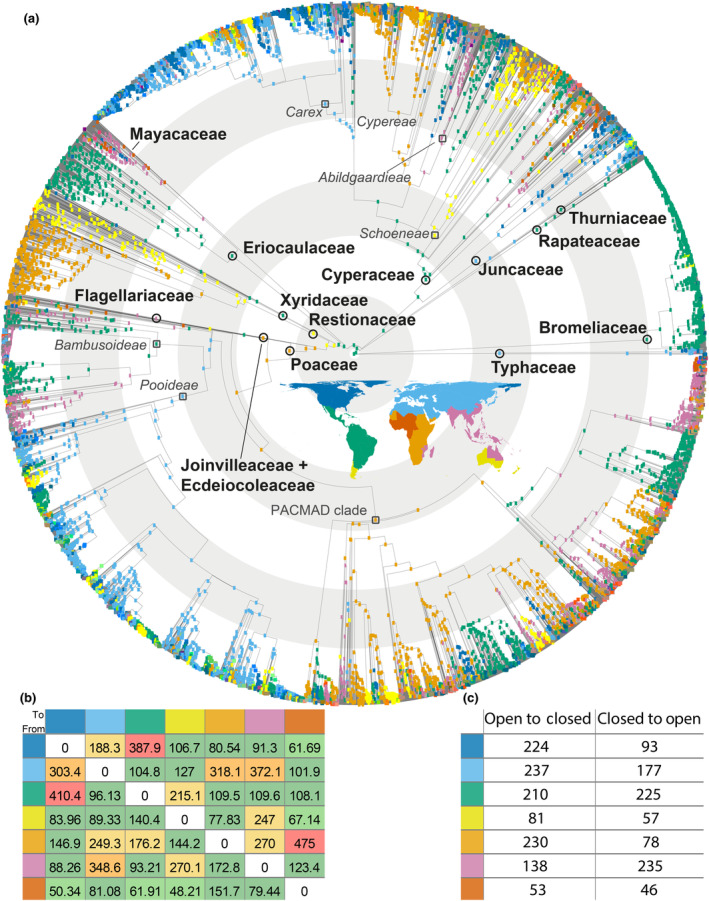
Ancestral area reconstruction within Poales based on seven regions, obtained using the dispersal–extinction–cladogenesis (DEC) model in BioGeoBEARS (a). A global map showing colours corresponding to the seven defined areas for the BioGeoBEARS analysis is in the centre of the phylogenetic reconstruction. The crown nodes of the families within Poales are shown with black circles, whereas dark grey squares are used to depict lineages important for the study's interpretations. Concentric light grey/white rings underlying the phylogeny indicate time slots of 20 Myr intervals. Note that Joinvilleaceae and Ecdeiocoleaceae are depicted together for visual purposes. Number of dispersal events between the seven regions inferred using Biogeographical Stochastic Mapping on the DEC model (b), and (c) number of transitions between open and closed habitats inferred to have occurred within each of the seven areas, calculated through comparison of best fitting corHMM models and historical biogeographical estimates. The colours of row and column labels in (b) and row in (c) correspond to those on inset map in (a).
Poaceae and Cyperaceae experienced a several million‐year lag before co‐occurring in any phyloregion (Figs 2, 3). This pattern of concurrent but spatially separated diversification is repeated for main lineages within these families as well. In the Paleocene, the grass clades Bambusoideae, PACMAD, and Pooideae originate allopatrically in the Neotropics, Afrotropics, and Eurasia, respectively. In the Eocene, the sedge clades Abildgaardieae, Cypereae, Carex and Schoeneae originate in the Indomalayan tropics, Afrotropics, Palearctic, and Austral region, respectively.
Dispersal across all phyloregions has occurred many times throughout the evolution of Poales, most frequently between the Neartic and Neotropics and between the Palearctic and Paleotropics (Fig. 3b). Eurasia is a strong one‐way source to North America, tropical Africa and tropical Asia, while equatorial Africa is a notable recipient of lineages from Sub‐Saharan Africa (Fig. 3a,b). A relatively high number of transitions between open and closed habitats occurred in the Neotropics and Eurasia compared to equatorial Africa and the Austral region (Fig. 3a,c). Transitions from open to closed are more frequent than the opposite for the Neoarctic and Sub‐Saharan Africa, whereas closed to open transitions are more numerous for the Indomalayan region (Fig. 3a,c).
The asymmetrical (“all‐rates‐different”) with hidden states model with two categories is the best fit corHMM model of evolution for open/closed habitats (AIC = 8814.2; Fig. 4; Table S5). An open habitat (Fig. 4) is inferred at the root of Poales and most families, with shift to closed habitat near zero (0.000000001 transitions per million years (Myr) in R2 (Regime rate 2)). However, there are rapid rate transitions between open and closed (0.31 transitions/Myr in R1 [Regime rate 1]) and closed to open (0.51 transitions/Myr in R1) habitats among Bromeliaceae, Cyperaceae (Carex clade) and Poaceae (Bambusoideae clade), occurring predominantly during the Miocene.
Spatial phylogenetics
The species in our phylogeny were generally representative of the cosmopolitan distribution of Poales, except for the underrepresentation of tropical areas in South America, India and parts of China (Fig. S2). Across Poales and in all families except Bromeliaceae, phylogenetic diversity is substantially higher in open habitats than closed habitats in all phyloregions (Figs 5a, S3), with the highest values attributed to open habitat species in southern Africa, western Australia and northern South America. High open habitat PD is driven by Cyperaceae, Poaceae and Restionaceae in southern Africa; Cyperaceae, Poaceae and Restionaceae in western Australia; and by Bromeliaceae, Cyperaceae, Eriocaulaceae and Poaceae in northern South America (Figs 3, 5, S3). Although closed habitat PD is lower overall than open habitat PD, northern South America is a high centre of diversity of these species (Fig. 3b), with high representation of Bromeliaceae, Cyperaceae and Poaceae. Despite the widespread availability of forested habitats throughout the Holarctic, these regions have low PD of closed habitat Poales. Those species that do occur in closed habitats in the Holarctic represent only Cyperaceae, Juncaceae and Poaceae (Fig. S3). With the exception of Juncaceae, closed habitat PD is higher in all major families south of the Tropic of Cancer (Fig. S3).
Fig. 5.
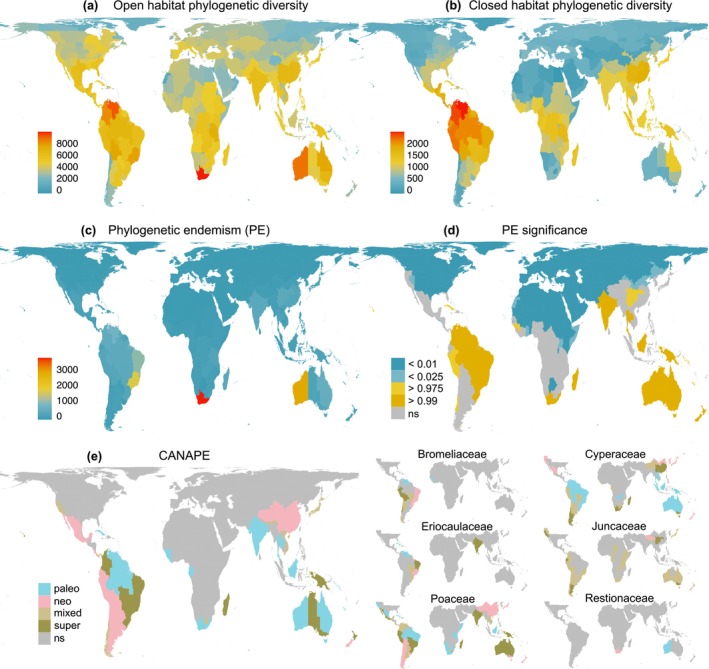
Biodiversity patterns in Poales represented by Faith's phylogenetic diversity (PD) for open habitat species (a) and PD for closed habitat species (b); (c) Rosauer's phylogenetic endemism (PE) and (d) PE significance; and (e) centres of palaeo‐ and neo‐endemism determined using CANAPE for all Poales (left) and the six most speciose families (right). High PE significance indicates that the region has an overrepresentation of short, rare branches. Low PE significance indicates that short, rare branches are underrepresented. ns, not significant.
By far, southern Africa displays the highest PE in Poales (Fig. 5c), driven by high endemism of Cyperaceae, Poaceae and Restionaceae (Fig. S4). Phylogenetic endemicity is also high in Western Australia, again because of restricted lineages from the same three families, and southeastern Brazil, largely due to Bromeliaceae and Eriocaulaceae (Figs 5c, S4). The Neotropical and Paleotropical regions are clearly centres of significant PE, with nearly all Holarctic regions exhibiting significantly low PE and many botanical countries in the tropics and subtropics exhibiting significantly high PE. California is the only north temperate zone with significantly high PE, driven by Poaceae and Juncaceae (Figs 5c, S4). Overall, family‐level patterns of significant PE match those of Poales, with low PE in the North and high PE in the South, except for Typhaceae which tends to have low PE in most of its range.
Categorical analysis of endemism (CANAPE; Fig. 5e) shows a general contrasting pattern for Poales between Holarctic (mostly non‐significant) and Holotropic + Austral (significant) regions. Palaeo‐endemism in Poales was supported in northern South America, southern Africa and Australia – a pattern also shared with Poaceae (including Tanzania, CA) and Cyperaceae, whereas palaeo‐endemism is only evident in Australia for the Cyperaceae and Restionaceae. Neo‐endemism is observed in western South America (Andean mountains) in the Poales and Poaceae, but in the eastern South American region (Brazil) for Bromeliaceae and Eriocaulaceae; in most of East Asia for the order and families Poaceae and Cyperaceae; and in southern Africa for the Restionaceae. A mixture of both palaeo‐ and neo‐endemism is observed in Australia and South America for both the Poales and Poaceae. Though areas of endemism in Poaceae and Cyperaceae often co‐occur, frequently centres of palaeo‐ or neo‐endemism in one family are centres of mixed/super endemism in the other (e.g. Andean Mountains, Australia, southeast Brazil, eastern China, southern Africa).
Discussion
The key outcome of this comprehensive study of Poales is an emerging picture of parallel evolution – in space, through time, across lineages – resulting in the global assembly of open habitats, with notable, spatially and phylogenetically restricted divergence into closed habitats. Our analyses show that each family exhibits a contrasting historical biogeography and pattern of habitat transition, which manifest in unique spatial patterns of assembly. And yet, patterns of evolution and assembly are repeated in most families.
The biogeography and regionalisation of Poales
The Poales represent over 120 Myr of diversification, with an estimated Cretaceous origin in Western Gondwana (Fig. 4a). Consistent with previous phylogenetic studies, our model places the Poaceae origin in the Afrotropics with eventual migration to the Neotropics in the Eocene (Bouchenak‐Khelladi et al., 2010; Gallaher et al., 2022). Meanwhile, Cyperaceae originates in the Neotropics and quickly shifts to Patagonia‐Australia before establishing in Africa in the Paleocene (Spalink et al., 2016; Larridon et al., 2021a). The earliest Cyperaceae fossils also place extinct Cyperaceae lineages in Europe by the Paleogene (Smith et al., 2009). Consistent with our hypotheses, the subsequent global dominance of these Poaceae and Cyperaceae clades is a tale of parallel diversification with repeated spatial convergence of distantly related lineages, which is also observed in transitions between open and closed habitats (Fig. 4) and has clear impacts on spatial patterns of phylogenetic endemism.
In contrast to Poaceae and Cyperaceae, the other families have experienced more limited diversification, either spatially or in terms of species richness. The oldest of these is Restionaceae, which originated in Western Australia in the Late Cretaceous and dispersed to southern Africa in the Eocene (Fig. 3a; Linder et al., 2003). This C3 family of predominantly perennials remains restricted to open habitats in these two regions, where they have evolved strategies to withstand frequent fires (reseeder/resprouter; Litsios et al., 2014) and thrive on oligotrophic soils (cluster roots; Lambers et al., 2006), traits only shared by the Abildgaardieae, Rhynchosporeae and Schoeneae (Cyperaceae; Barrett, 2013; Fidelis et al., 2019; Pilon et al., 2023) and lacking in the Poaceae. Typhaceae and Juncaceae, both of Palearctic origin near the KP boundary, are now cosmopolitan but remain species poor. Eriocaulaceae originated in the Neotropics at about the same time, and only a few lineages in the family have diversified outside this ancestral zone. Bromeliaceae are perhaps the most unique among the Poales, diversifying nearly exclusively in Central‐ and South America and with suites of traits not found in other families, such as epiphytism and lithophytism, traits encountered in the Poales outside the Bromeliaceae only among the poikilohydric clade of Cyperaceae (Trilepideae; Muasya et al., 2010; Porembski et al., 2021).
These historical processes have resulted in substantial spatial structure to regionalisation in Poales, with lineages forming 13 distinct phyloregions (Fig. 2a) clustered into three floristic kingdoms (sensu Carta et al., 2022; Fig. S5). Despite the propensity for long‐distance dispersal in many Poales groups (Linder et al., 2018; Martín‐Bravo et al., 2019; Benítez‐Benítez et al., 2021; Larridon et al., 2021a; Gallaher et al., 2022), regionalisation in this clade largely mirrors that of all vascular plants, where the tectonic legacy of Gondwana and Laurasia persists in determining, at least in part, the distribution of lineages (Takhtajan, 1986; Carta et al., 2022). In Poales, this is evident in northern temperate regions being distinct from those in the tropical south, and a united Austral‐Patagonian (holantarctic, sensu Takhtajan, 1986) region reflecting the historical connection of these now‐distance land masses. Temperate zones of both hemispheres share more lineages than they do with tropical zones, and the American tropics are phylogenetically distinct from the Paleotropics (Fig. S5). The unified North–South Temperate floristic kingdom may be partly due to bipolar disjunctions within species (at least 14) and genera (at least eight) in Poales (Villaverde et al., 2017). Likely, a more important driver of this pattern is the ecological filtering of lineages with respect to traits that evolved earlier in the diversification of Poales. These would include traits such as frost hardiness and seasonality tolerance in Juncaceae, Typhaceae and select Poaceae and Cyperaceae clades (Vigeland et al., 2013; Martín‐Bravo et al., 2019; Ambroise et al., 2020; Schubert et al., 2020), and edaphic specificity coupled with functional traits associated with adaptations to temperature and water extremes, as well as disturbance regimes (e.g. C4, CAM, silica, tannins, epiphytism; Givnish et al., 2011, 2014; Linder et al., 2018) across Poales. The relatively few lineages that have evolved tolerance to frost and strong climatic seasonality have diversified extensively, virtually in all habitable places where these conditions persist (Fig. 2). The phylogenetic differentiation of the American‐ and Palaeo‐ tropical kingdoms has roots deep in the Poales phylogeny. The Neotropical origin of Cyperaceae and Afrotropical origin of Poaceae, the restriction of Eriocaulaceae, Bromeliaceae, and Rapateaceae to the Neotropics, and that of Restionaceae to austral regions (Australia, New Zealand, southern Africa and Patagonia) drive strong phylogenetic beta‐diversity across the tropics.
Evolutionary parallelism in open and closed habitats
The parallel evolution of open and closed lineages in Poales is most evident in Poaceae and Cyperaceae, which collectively represent c. 74% of species richness in the order and the majority of open‐habitat PD (Fig. S3). Both families encompass two species‐rich lineages (in Cyperaceae: Carex and Cypereae; in Poaceae: PACMAD and Pooideae), each of which (except Pooideae) is inferred to have an open‐habitat ancestor (Fig. 4). The exclusively C3 Carex and Pooideae are predominately temperate lineages, likely originating in eastern Asia (Fig. 3; Spalink et al., 2016; Martín‐Bravo et al., 2019; Gallaher et al., 2022), while tropical Africa is inferred as the origin for the heavily C4 PACMAD and Cypereae clades (Figs 3a, 4). These species‐rich lineages evolved from C3 ancestors, acquiring cold tolerance (Carex, Pooideae; Vigeland et al., 2013; Martín‐Bravo et al., 2019; Schubert et al., 2020) and C4 photosynthesis (Cypereae, Abildgaardieae, PACMAD; Fig. 4) in parallel. The tempo of habitat transition has shifted from slow to fast multiple times (Fig. 4), most notably in Carex (> 2000 species; Larridon et al., 2021b), Bambusoideae (c. 1700 species; Soreng et al., 2022), and Bromeliaceae (c. 3700 species, Gouda & Butcher, 2023). Carex is the largest clade in the phylogeny in which fast transitions between open and closed and vice versa are quite frequent (Fig. 4). This may represent the most significant difference with Pooideae. Bambusoideae uniquely grow tall enough to reach the canopy (Soreng et al., 2015; Attigala et al., 2016). Bromeliaceae likely originated in open habitats, but like Carex, has rapidly and repeatedly shifted between both habitats (Fig. 4).
Ultimately, our analyses indicate that there was likely a high diversity in all phyloregional species pools from multiple families with beneficial adaptations for survival in open habitats before their expansion in the Miocene (Figs 3, 4; Edwards et al., 2010; Veldman et al., 2015). Consistent with the fossil record (Prasad et al., 2005; Vicentini et al., 2008), the origin of PACMAD (encompassing all known origins of C4 in Poaceae; Edwards, 2019) and Abildgaardieae and Cypereae (majority of C4 Cyperaceae) is placed in the Eocene and Palaeocene (Fig. 3; Prasad et al., 2011; Gallaher et al., 2022), but most of the diversification in these clades coincides and is possibly linked with the low CO2 concentrations, cooler, drier conditions, and increased fire activity and herbivory during the Miocene (Osborne & Freckleton, 2009; Edwards & Smith, 2010; Strömberg, 2011; Maurin et al., 2014; Sage et al., 2018; Peppe et al., 2023). Diversification of most major Poales families occurred in all phyloregions during this time (Fig. 2).
Spatial phylogenetics of Poales
The spatial structure of phylogenetic diversity at the resolution presented here (Figs 5, S4, S5) results from historical biogeographical and ecological processes rather than local contemporary ecological processes (Meynard et al., 2013; Ross et al., 2021). The observed diversity patterns are complex (Fig. 5). Each family exhibits peak PD (Fig. 2) in a different botanical country – usually, but not always in their phyloregion of estimated origin (Fig. 3a). The PD of the order as a whole, and each family independently, defies a traditional latitudinal diversity gradient (Kreft & Jetz, 2007). Though this is the dominant trend in speciose open habitat lineages, the PD of closed habitat lineages peaks in equatorial forests and decreases poleward (Fig. 5a,b). These patterns reflect the tremendous diversification of Poales in open habitats around the world (Figs 2, 3, S4), with much more limited diversification of closed habitat lineages outside ancestral ranges.
Patterns of diversity in Poales generally align with our stated hypotheses. Phylogenetic diversity peaks in the tropics, where most families originated. Hotspots of PE (Fig. 5c–e) are almost strictly a southern hemispheric phenomenon, and apart from California in North America, never occur north of 33°N. This pattern is likely driven both by the presence of spatially restricted families in the Southern Hemisphere (e.g. Bromeliaceae, Restionaceae and Rapateaceae; Fig. 2b) and the propensity of lineages to migrate across the Holarctic (Donoghue & Smith, 2004).
To our knowledge, this is the first study coupling CANAPE with historical biogeographical estimations on a global scale. We find contrasting patterns of palaeo‐ and neo‐endemism clearly reflect historical biogeographical processes at the family level (Fig. 5e). In Bromeliaceae, centres of palaeoendemism occur in the Guayana Shield – the site of putative origin of the family – whereas centres of neoendemism are restricted to the Brazilian Shield, where Bromelioideae subsequently radiated (Givnish et al., 2011). Eriocaulaceae mirror this pattern (Andrino et al., 2023), with the added area of super‐endemism in India, where nearly 20% of the family occurs in the Western Ghats region (Sunil et al., 2015). In Restionaceae, Western Australia, the ancestral home of the family, hosts palaeoendemics, while neoendemics are restricted to South Africa (Linder et al., 2003). And finally, Poaceae and Cyperaceae show high levels of both endemism types around the world, reflecting their rapid migration early in their evolutionary history (Fig. 3) and in situ diversification across phyloregions (Fig. 2). In both families, tropical savannas and forested regions primarily harbour palaeoendemics, whereas more recent diversification is found frequently on islands – both young and old – and mountains. Significant palaeoendemism is observed within old landscapes with buffered climates (South America, southern Africa, India, and Australia; Hopper et al., 2016), while significant neoendemism is predominantly within regions that have experienced Miocene geomorphological evolution – especially in Andean (Gregory‐Wodzicki, 2000) and Himalayan mountains (Wang et al., 2022). Areas containing a mixture of both neo‐ and palaeoendemism are observed in regions where old and young landscapes are interspersed (Hopper et al., 2016; Vasconcelos et al., 2020; Barros‐Souza & Borges, 2023).
Conclusions
The biogeography of individual families in the Poales is distinct from early in their evolution, such that few major clades originating in approximate synchrony do so in the same places. This is particularly evident in Poaceae and Cyperaceae, whose evolution is like two dancers on a global stage – a biogeo(choreo)graphy – characterised by parallel movement through a shared temporality but with varying tempos, spatial staging, and ecological rhythms, ultimately resulting in a final tableau of global dominance. Both families originate in the late Cretaceous, but on either side of the widening Atlantic Ocean, dispersing and evolving in parallel to achieve cosmopolitan distribution. In contrast, the other families have experienced more limited, spatially restricted diversification. We identify latitudinal and longitudinal patterns of diversification, most evident in the phylogenetic distinctiveness of the temperate regions and also in the American tropics separating from the palaeotropics, with highest endemism found in the Neo‐ and Paleotropics. Parallel evolution is observed in habitat transition, whose tempo has shifted from slow to fast multiple times especially since the Eocene, driven by repeated evolution of traits enabling colonisation of open and closed habitats in different latitudes.
Competing interests
None declared.
Author contributions
AMM, TLE, DS and IL conceived the study. AMM, TLE, ME, DS, IL, JH, RLB, SM‐B, JIM‐C, CGM, ACM, KJR‐S, DAZ, COA, DC, MSV, KLW and DAS collected data, while TLE, DS, JH, ARZ and ME conducted the analyses. AMM, TLE and DS wrote the manuscript with input from all co‐authors. JIM‐C, DS, ME and RLB created the figures. TLE and DS contributed equally to this work.
Supporting information
Fig. S1 Ancestral area reconstruction within Poales based on seven regions, obtained using the DIVA model in BioGeoBEARS.
Fig. S2 Number of species of Poales missing from the phylogenetic data set compared to the number listed in the World Checklist of Vascular Plants as of 28 February 2022, mapped per botanical region.
Fig. S3 Phylogenetic diversity of the six largest Poales families categorised into open and closed habitats.
Fig. S4 Phylogenetic endemicity mapped per botanical region for Poales and eight families with the highest number of species in the data set.
Fig. S5 Poales botanical regions grouped into three ‘floristic kingdoms’ based on phylogenetic beta‐diversity, indicated by different colours and numbers.
Notes S1 Methodological detail on sequence assembly and tree construction.
Notes S2 Justification for selecting dispersal–extinction–cladogeneis model of ancestral estimation.
Table S1 Sequences for the phylogenetic reconstruction.
Table S2 Calibrations used in the treePL (Smith & O'Meara, 2012) configuration file.
Table S3 Taxa included in this study, showing habitat scoring across the Poales.
Table S4 Comparison of six ancestral area reconstruction models based on BioGeoBEARS analyses for Poales.
Table S5 Results from corHMM ancestral state reconstructions.
Please note: Wiley is not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
The authors acknowledge the team who delivered the WCVP on which many of the analyses performed in this study are based. This work was partly funded by grants from the Calleva Foundation to the Plant and Fungal Trees of Life Project (PAFTOL) at the Royal Botanic Gardens, Kew. The authors are thankful for the Research/Scientific Computing teams at The James Hutton Institute and NIAB for providing computational resources used for the phylogenomic analyses performed in the ‘UK's Crop Diversity Bioinformatics HPC’ (BBSRC grant BB/S019669/1). Computational resources were also supplied by the project ‘e‐Infrastruktura CZ’ (e‐INFRA CZ ID:90140) supported by the Ministry of Education, Youth and Sports of the Czech Republic. Lizzy Wenk extracted data from AusTraits. This work was supported in part by USDA National Institute of Food and Agriculture (McIntire Stennis Project 1018692) and NSF #1902064 to DS; UNAM–DGAPA–PAPIIT (No. IA202319), ‘Investigación Científica Básica’ CONAHCYT (No. 286249) to CGM; MICINN‐AEI to ME (PID2021‐122715NB‐I00) and SM‐B (PID2020‐113897GB‐I00). KR thanks DGAPA‐UNAM for a postdoctoral grant (2022). ARZ and JH were each funded by a ‘Future Leader in Plant and Fungal Science’ fellowship from the Royal Botanic Gardens, Kew.
Data availability
The data sets analysed in this study are available as Supporting Information. The new Angiosperms353 raw reads are deposited in the European Nucleotide Archive (PRJEB35285).
References
- Ambroise V, Legay S, Guerriero G, Hausman JF, Cuypers A, Sergeant K. 2020. The roots of plant frost hardiness and tolerance. Plant and Cell Physiology 61: 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrino CO, Costa FN, Simon MF, Missagia RV, Sano PT. 2023. Eriocaulaceae: a new classification system based on morphological evolution and molecular evidence. Taxon 72: 515–549. [Google Scholar]
- APG IV . 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. [Google Scholar]
- Attigala L, Wysocki WP, Duvall MR, Clarke LG. 2016. Phylogenetic estimation and morphological evolution of Arundinarieae (Bambusoideae: Poaceae) based on plastome phylogenomic analysis. Molecular Phylogenetics and Evolution 101: 111–121. [DOI] [PubMed] [Google Scholar]
- Bailey SF, Blanquart F, Bataillon T, Rees K. 2017. What drives parallel evolution? How population size and mutational variation contribute to repeated evolution. BioEssays 39: 1–9. [DOI] [PubMed] [Google Scholar]
- Baker WJ, Bailey P, Barber V, Barker A, Bellot S, Bishop D, Botigué LR, Brewer G, Carruthers T, Clarkson JJ et al. 2022. A comprehensive phylogenomic platform for exploring the Angiosperm tree of life. Systematic Biology 71: 301–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett RL. 2013. Ecological importance of sedges: a survey of the Australasian Cyperaceae genus Lepidosperma . Annals of Botany 111: 499–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros‐Souza Y, Borges LM. 2023. Spatial‐and lineage‐dependent processes underpin floristic assembly in the megadiverse Eastern South American mountains. Journal of Biogeography 50: 302–315. [Google Scholar]
- Beaulieu JM, O'Meara BC. 2018. Can we build it? Yes we can, but should we use it? Assessing the quality and value of a very large phylogeny of campanulid angiosperms. American Journal of Botany 105: 417–432. [DOI] [PubMed] [Google Scholar]
- Benítez‐Benítez C, Martín‐Bravo S, Bjorå CS, Gebauer S, Hipp AL, Hoffmann MH, Luceño M, Pedersen TM, Reznicek A, Roalson E et al. 2021. Geographical vs ecological diversification in Carex section Phacocystis (Cyperaceae): patterns hidden behind a twisted taxonomy. Journal of Systematics and Evolution 59: 642–667. [Google Scholar]
- Benson DA, Karsch‐Mizrachi I, Lipman DJ, Ostell J, Sayers EW. 2010. GenBank. Nucleic Acids Research 38: D46–D51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick DI, Barrett RDH, Oke KB, Rennison DJ, Stuart YE. 2018. (Non) parallel evolution. Annual Review of Ecology, Evolution, and Systematics 49: 303–330. [Google Scholar]
- Bond WJ. 2019. Open ecosystems: ecology and evolution beyond the forest edge. Oxford, UK: Oxford University Press. [Google Scholar]
- Bond WJ. 2022. Out of the shadows: ecology of open ecosystems. Plant Ecology and Diversity 14: 205–222. [Google Scholar]
- Bouchenak‐Khelladi Y, Muasya AM, Linder P. 2014. A revised evolutionary history of Poales: origins and diversification. Botanical Journal of the Linnaean Society 175: 4–16. [Google Scholar]
- Bouchenak‐Khelladi Y, Verboom GA, Savolainen V, Hodkinson TR. 2010. Biogeography of the grasses (Poaceae): a phylogenetic approach to reveal evolutionary history in geographical space and geological time. Botanical Journal of the Linnean Society 162: 543–557. [Google Scholar]
- Boyko JD, Beaulieu JM. 2021. Generalized hidden Markov models for phylogenetic comparative data sets. Methods in Ecology and Evolution 12: 468–478. [Google Scholar]
- Brummitt RK, Pando F, Hollis S, Brummitt N. 2001. World geographical scheme for recording plant distributions, 2nd ed. Pittsburgh, PA, USA: Hunt Institute for Botanical Documentation, Carnegie‐Mellon University. [Google Scholar]
- Buisson E, Archibald S, Fidelis A, Suding KN. 2022. Ancient grasslands guide ambitious goals in grassland restoration. Science 377: 594–598. [DOI] [PubMed] [Google Scholar]
- Carta A, Peruzzi L, Ramírez‐Barahona S. 2022. A global phylogenetic regionalization of vascular plants reveals a deep split between Gondwanan and Laurasian biotas. New Phytologist 233: 1494–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BE, Misiewicz TM, Mishler BD. 2022. Spatial phylogenetic patterns in the North American moss flora are shaped by history and climate. Journal of Biogeography 49: 1327–1338. [Google Scholar]
- Chamberlain S, Szocs E. 2013. taxize – taxonomic search and retrieval in R. F1000Research . [WWW document] URL https://f1000research.com/articles/2‐191/v2 [accessed 11 April 2022]. [DOI] [PMC free article] [PubMed]
- Dahlgren RM, Clifford HT, Yeo PF. 1985. The families of the monocotyledons. Berlin, Germany: Springer‐Verlag. [Google Scholar]
- Daru BH, Elliott TL, Park DS, Davies TJ. 2017. Understanding the processes underpinning patterns of phylogenetic regionalization. Trends in Ecology & Evolution 32: 845–860. [DOI] [PubMed] [Google Scholar]
- Daru BH, Farooq H, Antonelli A, Faurby S. 2020a. Endemism patterns are scale dependent. Nature Communications 11: 2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daru BH, Karunarathne P, Schliep K. 2020b. phyloregion: R package for biogeographic regionalization and macroecology. Methods in Ecology and Evolution 11: 1483–1491. [Google Scholar]
- Dinerstein E, Olson D, Joshi A, Vynne C, Burgess ND, Wikramanayake E, Hahn N, Palminteri S, Hedao P, Noss R et al. 2017. An ecoregion‐based approach to protecting half the terrestrial realm. Bioscience 67: 534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue MJ, Smith SA. 2004. Patterns in the assembly of temperate forests around the Northern Hemisphere. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 359: 1633–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán‐Castillo M, Hudson A, Wilson Y, Field DL, Twyford AD. 2022. A phylogeny of Antirrhinum reveals parallel evolution of alpine morphology. New Phytologist 233: 1426–1439. [DOI] [PubMed] [Google Scholar]
- Edwards EJ. 2019. Evolutionary trajectories, accessibility and other metaphors: the case of C4 and CAM photosynthesis. New Phytologist 223: 1742–1755. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Osborne CP, Strömberg CAE, Smith SA, C4 Grasses Consortium , Bond WJ, Christin P‐A, Cousins AB, Duvall MR, Fox DL et al. 2010. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328: 587–591. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Smith SA. 2010. Phylogenetic analyses reveal the shady history of C4 grasses. Proceedings of the National Academy of Sciences, USA 107: 2532–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott TL, Zedek F, Barrett RL, Bruhl JJ, Escudero M, Hroudová Z, Joly S, Larridon I, Luceño M, Márquez‐Corro JI et al. 2022. Chromosome size matters: genome evolution in the cyperid clade. Annals of Botany 130: 999–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith DP. 1992. Conservation evaluation and phylogenetic diversity. Biological Conservation 61: 1–10. [Google Scholar]
- Fidelis A, Rosalem P, Zanzarini V, Camargos LS, Martins AR. 2019. From ashes to flowers: a savanna sedge initiates flowers 24 h after fire. Ecology 100: e02648. [DOI] [PubMed] [Google Scholar]
- Folk RA, Sun M, Soltis PS, Smith SA, Soltis DE, Guralnick RP. 2018. Challenges of comprehensive taxon sampling in comparative biology: wrestling with rosids. American Journal of Botany 105: 433–445. [DOI] [PubMed] [Google Scholar]
- Gallaher TJ, Peterson PM, Soreng RJ, Zuloaga FO, Li DZ, Clark LG, Tyrrell CD, Welker AD, Kellogg EA, Teisher JK. 2022. Grasses through space and time: an overview of the biogeographical and macroevolutionary history of Poaceae. Journal of Systematics and Evolution 60: 522–569. [Google Scholar]
- Givnish TJ, Barfuss MHJ, van Ee B, Riina R, Schulte K, Horres R, Gonsiska PA, Jabaily R, Crayn DM, Smith JAC et al. 2011. Phylogeny, adaptive radiation, and historical biogeography in Bromeliaceae: insights from an eight‐locus plastid phylogeny. American Journal of Botany 98: 872–895. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Barfuss MHJ, Van Ee B, Riina R, Schulte K, Horres R, Gonsiska PA, Jabaily RS, Crayn DM, Smith JAC et al. 2014. Adaptive radiation, correlated and contingent evolution, and net species diversification in Bromeliaceae. Molecular Phylogenetics and Evolution 71: 55–78. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Evans TM, Zjhra ML, Patterson TB, Berry PE, Sytsma KJ. 2000. Molecular evolution, adaptive radiation, and geographic diversification in the amphiatlantic family Rapateaceae: evidence from ndhF sequences and morphology. Evolution 54: 1915–1937. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Zuluaga A, Spalink D, Soto Gomez M, Lam VK, Saarela JM, Sass C, Iles WJ, De Sousa DJ, Leebens‐Mack J et al. 2018. Monocot plastid phylogenomics, timeline, net rates of species diversification, the power of multi‐gene analyses, and a functional model for the origin of monocots. American Journal of Botany 105: 1888–1910. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Orozco CE, Pollock LJ, Thornhill AH, Mishler BD, Knerr N, Laffan SW, Miller JT, Rosauer DF, Faith DP, Nipperess DA et al. 2016. Phylogenetic approaches reveal biodiversity threats under climate change. Nature Climate Change 6: 1110–1114. [Google Scholar]
- Gotelli NJ, Entsminger NJ. 2003. Swap algorithms in null model analysis. Ecology 84: 532–535. [Google Scholar]
- Gouda EJ, Butcher D. 2023. The new bromeliad taxon list, v.4. Utrecht, the Netherlands: University Botanic Gardens. [WWW document] URL https://bromeliad.nl/taxonlist/ [accessed 1 February 2023]. [Google Scholar]
- Govaerts R, ed. 2022. WCVP: world checklist of vascular plants. Kew, UK: Facilitated by the Royal Botanic Gardens. [WWW document] URL http://sftp.kew.org/pub/data‐repositories/WCVP/Special_Issue_28_Feb_2022/ [accessed 28 February 2022]. [Google Scholar]
- Gregory‐Wodzicki KM. 2000. Uplift history of the Central and Northern Andes: a review. Geological Society of America Bulletin 112: 1091–1105. [Google Scholar]
- Hartigan JA, Wong MA. 1979. Algorithm AS 136: a K‐means clustering algorithm. Applied Statistics 28: 100–108. [Google Scholar]
- Holt BG, Lessard JP, Borregaard MK, Fritz SA, Araújo MB, Dimitrov D, Fabre PH, Graham CH, Graves GR, Jønsson KA et al. 2013. An update of Wallace's zoogeographic regions of the world. Science 339: 74–78. [DOI] [PubMed] [Google Scholar]
- Hopper SD, Silveira FA, Fiedler PL. 2016. Biodiversity hotspots and Ocbil theory. Plant and Soil 403: 167–216. [Google Scholar]
- Johnson MG, Pokorny L, Dodsworth S, Botigue LR, Cowan RS, Devault A, Eiserhardt WL, Epitawalage N, Forest F, Kim JT et al. 2019. A universal probe set for targeted sequencing of 353 nuclear genes from any flowering plant designed using k‐medoids clustering. Systematic Biology 68: 594–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. Mafft multiple sequence alignment software v.7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kembel SW, Cowan PD, Helmus MR, Cornwell WL, Morlon H, Ackerly DD, Blomberg SP, Webb CO. 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26: 1463–1464. [DOI] [PubMed] [Google Scholar]
- Kreft H, Jetz W. 2007. Global patterns and determinants of vascular plant diversity. Proceedings of the National Academy of Sciences, USA 104: 5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. 2006. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany 98: 693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larridon I, Spalink D, Jiménez‐Mejías P, Márquez‐Corro JI, Martín‐Bravo S, Muasya M, Escudero M. 2021a. The evolutionary history of sedges (Cyperaceae) in Madagascar. Journal of Biogeography 48: 917–932. [Google Scholar]
- Larridon I, Zuntini AR, Léveillé‐Bourret E, Barrett RL, Starr JR, Muasya AM, Villaverde T, Bauters K, Brewer GE, Bruhl JJ et al. 2021b. A new classification of Cyperaceae (Poales) supported by phylogenomic data. Journal of Systematics and Evolution 59: 852–895. [Google Scholar]
- Larsson A. 2014. AliView: a fast and lightweight alignment viewer and editor for large data sets. Bioinformatics 30: 3276–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder HP, Eldenas P, Briggs BG. 2003. Contrasting patterns of radiation in African and Australian Restionaceae. Evolution 57: 2688–2702. [DOI] [PubMed] [Google Scholar]
- Linder HP, Lehmann CER, Archibald S, Osborne CP, Richardson DM. 2018. Global grass (Poaceae) success underpinned by traits facilitating colonization, persistence and habitat transformation. Biological Reviews 93: 1125–1144. [DOI] [PubMed] [Google Scholar]
- Linder HP, Rudall PJ. 2005. Evolutionary history of Poales. Annual Review of Ecology, Evolution, and Systematics 36: 107–124. [Google Scholar]
- Litsios G, Wüest RO, Kostikova A, Forest F, Lexer C, Linder HP, Pearman PB, Zimmermann NE, Salamin N. 2014. Effects of a fire response trait on diversification in replicated radiations. Evolution 68: 453–465. [DOI] [PubMed] [Google Scholar]
- Louca S, Pennell MW. 2020. Extant timetrees are consistent with a myriad of diversification histories. Nature 580: 502–505. [DOI] [PubMed] [Google Scholar]
- Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K. 2022. cluster: Cluster analysis basics and extensions. R package v.2.1.3 . [WWW document] URL https://CRAN.R‐project.org/package=cluster [accessed 1 May 2022].
- Mai U, Mirarab S. 2018. TreeShrink: fast and accurate detection of outlier long branches in collections of phylogenetic trees. BMC Genomics 19: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín‐Bravo S, Jiménez‐Mejías P, Villaverde T, Escudero M, Hahn M, Spalink D, Roalson EH, Hipp AL, Global Carex Group . 2019. A tale of worldwide success: behind the scenes of Carex (Cyperaceae) biogeography and diversification. Journal of Systematics and Evolution 57: 695–718. [Google Scholar]
- Matzke NJ. 2013. Probabilistic historical biogeography: new models for founder‐event speciation, imperfect detection, and fossils allow improved accuracy and model‐testing. Frontiers of Biogeography 5: 242–248. [Google Scholar]
- Matzke NJ. 2014. Model selection in historical biogeography reveals that founder‐event speciation is a crucial process in Island Clades. Systematic Biology 63: 951–970. [DOI] [PubMed] [Google Scholar]
- Maurin O, Davies TJ, Burrows JE, Daru BH, Yessoufou K, Muasya M, van der Bank M, Bond WJ. 2014. Savanna fire and the origins of the ‘underground forests’ of Africa. New Phytologist 204: 201–214. [DOI] [PubMed] [Google Scholar]
- Meynard CN, Lavergne S, Boulangeat I, Garraud L, Van Es J, Mouquet N, Thuiller W. 2013. Disentangling the drivers of metacommunity structure across spatial scales. Journal of Biogeography 40: 1560–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, Haeseler A, Lanfear R. 2020. IQ‐Tree 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37: 1530–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishler BD, Knerr N, González‐Orozco CE, Thornhill AH, Laffan SW, Miller JT. 2014. Phylogenetic measures of biodiversity and neo‐ and paleo‐endemism in Australian Acacia . Nature Communications 5: 4473. [DOI] [PubMed] [Google Scholar]
- Muasya AM, Harvey Y, Cheek M, Tah K, Simpson DA. 2010. Coleochloa domensis (Cyperaceae), a new epiphytic species from Cameroon. Kew Bulletin 65: 323–325. [Google Scholar]
- Nitta JH, Iwasaki W. 2021. canaper: categorical analysis of neo‐ and paleo‐endemism in R . doi: 10.5281/zenodo.5094032. [DOI]
- Nitta JH, Mishler BD, Iwasaki W, Ebihara A. 2022. Spatial phylogenetics of Japanese ferns: patterns, processes, and implications for conservation. American Journal of Botany 109: 727–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CP, Freckleton RP. 2009. Ecological selection pressures for C4 photosynthesis in the grasses. Proceedings of the Royal Society B: Biological Sciences 276: 1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppe DJ, Cote SM, Deino AL, Fox DL, Kingston JD, Kinyanjui RN, Lukens WE, MacLatchy LM, Novello A, Strömberg CA et al. 2023. Oldest evidence of abundant C4 grasses and habitat heterogeneity in eastern Africa. Science 380: 173–177. [DOI] [PubMed] [Google Scholar]
- Pilon NA, Freire CT, Oliveira‐Alves MJ, Oliveira RS. 2023. Speedy blooming in Cerrado after fire is not uncommon: new records of Cyperaceae species flowering 24 h after burning. Austral Ecology 48: 1042–1045. [Google Scholar]
- Porembski S, Rexroth J, Weising K, Bondi L, Mello‐Silva R, Centeno DC, Datar MN, Watve A, Thiombano A, Tindano E et al. 2021. An overview on desiccation‐tolerant mat‐forming monocotyledons on tropical inselbergs. Flora 285: 151953. [Google Scholar]
- Prasad V, Stromberg CA, Alimohammadian H, Sahni A. 2005. Dinosaur coprolites and the early evolution of grasses and grazers. Science 310: 1177–1180. [DOI] [PubMed] [Google Scholar]
- Prasad V, Strömberg CAE, Leaché AD, Samant B, Patnaik R, Tang L, Mohabey DM, Ge S, Sahni A. 2011. Late Cretaceous origin of the rice tribe provides evidence for early diversification in Poaceae. Nature Communications 2: 480. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [WWW document] URL https://www.R‐project.org/ [accessed 1 May 2022]. [Google Scholar]
- Ratnam J, Bond WJ, Fensham RJ, Hoffmann WA, Archibald S, Lehmann CER, Anderson MT, Higgins SI, Sankaran M. 2011. When is a ‘forest’ a savanna, and why does it matter? Global Ecology and Biogeography 20: 653–660. [Google Scholar]
- Ree RH, Moore BR, Webb CO, Donoghue MJ. 2005. A likelihood framework for inferring the evolution of geographic range on phylogenetic trees. Evolution 59: 2299–2311. [PubMed] [Google Scholar]
- Ree RH, Sanmartín I. 2018. Conceptual and statistical problems with the DEC+ J model of founder‐event speciation and its comparison with DEC via model selection. Journal of Biogeography 45: 741–749. [Google Scholar]
- Ree RH, Smith SA. 2008. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology 57: 4–14. [DOI] [PubMed] [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Ricklefs RE, Renner SS. 1994. Species richness within families of flowering plants. Evolution 48: 1619–1636. [DOI] [PubMed] [Google Scholar]
- Rosauer D, Laffan SW, Crisp MD, Donnellan SC, Cook LG. 2009. Phylogenetic endemism: a new approach for identifying geographical concentrations of evolutionary history. Molecular Ecology 18: 4061–4072. [DOI] [PubMed] [Google Scholar]
- Ross S‐J, Suzuki Y, Kondoh M, Suzuki K, Villa Martín P, Dornelas M. 2021. Illuminating the intrinsic and extrinsic drivers of ecological stability across scales. Ecological Research 36: 364–378. [Google Scholar]
- Rousseeuw PJ. 1987. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. Journal of Computational and Applied Mathematics 20: 53–65. [Google Scholar]
- Sage RF, Monson RK, Ehleringer JR, Adachi S, Pearcy RW. 2018. Some like it hot: the physiological ecology of C4 plant evolution. Oecologia 187: 941–966. [DOI] [PubMed] [Google Scholar]
- Salvador S, Chan P. 2004. Determining the number of clusters/segments in hierarchical clustering/segmentation algorithms. In: Proceedings of the sixteenth IEEE international conference on tools with artificial intelligence. Piscataway, NS, USA: Institute of Electrical and Electronics Engineers, 576–584. [Google Scholar]
- Sanbonmatsu KK, Spalink D. 2022. A global analysis of mosses reveals low phylogenetic endemism and highlights the importance of long‐distance dispersal. Journal of Biogeography 49: 654–667. [Google Scholar]
- Schubert M, Humphreys AM, Lindberg CL, Preston JC, Fjellheim S. 2020. To coldly go where no grass has gone before: a multidisciplinary review of cold adaptation in Poaceae. Annual Plant Reviews 3: 523–562. [Google Scholar]
- Sechrest W, Brooks TM, da Fonseca GAB, Konstant WR, Mittermeier RA, Purvis A, Rylands AB, Gittleman JL. 2002. Hotspots and the conservation of evolutionary history. Proceedings of the National Academy of Sciences, USA 99: 2067–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Brown JW, Walker JF. 2018. So many genes, so little time: a practical approach to divergence‐time estimation in the genomic era. PLoS ONE 13: e0197433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, O'Meara BC. 2012. treePL: divergence time estimation using penalized likelihood for large phylogenies. Bioinformatics 28: 2689–2690. [DOI] [PubMed] [Google Scholar]
- Smith SY, Collinson ME, Simpson DA, Rudall PJ, Marone F, Stampanoni M. 2009. Elucidating the affinities and habitat of ancient, widespread Cyperaceae: Volkeria messelensis gen. et sp. nov., a fossil mapanioid sedge from the Eocene of Europe. American Journal of Botany 96: 1506–1518. [DOI] [PubMed] [Google Scholar]
- Solofondranohatra CL, Vorontsova MS, Hempson GP, Hackel J, Cable S, Jand V, Lehmann CER. 2020. Fire and grazing determined grasslands of central Madagascar represent ancient assemblages. Proceedings of the Royal Society B: Biological Sciences 287: 20200598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreng RJ, Peterson PM, Romaschenko K, Davidse G, Zuloaga FO, Judziewicz EJ, Filgueiras TS, Davis JI, Morrone O. 2015. A worldwide phylogenetic classification of the Poaceae (Gramineae). Journal of Systematics and Evolution 53: 117–137. [Google Scholar]
- Soreng RJ, Peterson PM, Zuloaga RO, Romaschenko K, Clarke LG, Teisher JK, Gillespie LJ, Barberá P, Welker CAD, Kellogg EA. 2022. A worldwide phylogenetic classification of the Poaceae (Gramineae) III: an update. Journal of Systematics and Evolution 60: 476–521. [Google Scholar]
- Spalink D, Drew BT, Pace MC, Zaborsky JG, Starr JR, Cameron KM, Givnish TJ, Sytsma KJ. 2016. Biogeography of the cosmopolitan sedges (Cyperaceae) and the area‐richness correlation in plants. Journal of Biogeography 43: 1893–1904. [Google Scholar]
- Spalink D, MacKay R, Sytsma KJ. 2019. Phylogeography, population genetics and distribution modelling reveal vulnerability of Scirpus longii (Cyperaceae) and the Atlantic Coastal Plain Flora to climate change. Molecular Ecology 28: 2046–2061. [DOI] [PubMed] [Google Scholar]
- Spalink D, Pender J, Escudero M, Hipp AL, Roalson EH, Starr JR, Waterway MJ, Bohs L, Sytsma KJ. 2018. The spatial structure of phylogenetic and functional diversity in the United States and Canada: an example using the sedge family (Cyperaceae). Journal of Systematics and Evolution 56: 449–465. [Google Scholar]
- Stamatakis A. 2014. RAxML v.8: a tool for phylogenetic analysis and post‐analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömberg CAE. 2011. Evolution of grasses and grassland ecosystems. Annual Review of Earth and Planetary Sciences 39: 517–544. [Google Scholar]
- Strona G, Nappo D, Boccacci F, Fattorini S, San‐Miguel‐Ayanz J. 2014. A fast and unbiased procedure to randomize ecological binary matrices with fixed row and column totals. Nature Communications 5: 4114. [DOI] [PubMed] [Google Scholar]
- Sunil CN, Ratheesh Narayanan MK, Sivadasan M, Alfarhan AH, Abdul JV. 2015. Eriocaulon vandaanamense sp. nov. (Eriocaulaceae) from Kerala, India. Nordic Journal of Botany 33: 155–158. [Google Scholar]
- Takhtajan A. 1986. Floristic regions of the world. Berkeley, CA, USA: University of California Press. [Google Scholar]
- Thornhill AH, Mishler BD, Knerr NJ, González‐Orozco CE, Costion CM, Crayn DM, Laffan SW, Miller JT. 2016. Continental‐scale spatial phylogenetics of Australian angiosperms provides insights into ecology, evolution and conservation. Journal of Biogeography 43: 2085–2098. [Google Scholar]
- Tibshirani R, Walther G, Hastie T. 2001. Estimating the number of data clusters via the Gap statistic. Journal of the Royal Statistical Society: Series B: Methodological 63: 411–423. [Google Scholar]
- Tucker CM, Cadotte MW, Carvalho SB, Davies TJ. 2017. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biological Review 92: 698–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos TN, Alcantara S, Andrino CO, Forest F, Reginato M, Simon MF, Pirani JR. 2020. Fast diversification through a mosaic of evolutionary histories characterizes the endemic flora of ancient Neotropical mountains. Proceedings of the Royal Society B: Biological Sciences 287: 20192933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman JW, Buisson E, Durigan G, Fernandes GW, Le Stradic S, Mahy G, Negreiros D, Overbeck GE, Veldman RG, Zaloumis NP. 2015. Toward an old‐growth concept for grasslands, savannas, and woodlands. Frontiers in Ecology and the Environment 13: 154–162. [Google Scholar]
- Vera‐Paz S, Granados Mendoza C, Díaz Contreras Díaz DD, Jost M, Salazar GA, Rossado AJ, Montes‐Azcué CA, Hernández‐Gutiérrez R, Magallón S, Sánchez‐González LA et al. 2023. Plastome phylogenomics reveals an early Pliocene North‐ and Central America colonization by long‐distance dispersal from South America of a highly diverse bromeliad lineage. Frontiers in Plant Science 14: 1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentini A, Barber JC, Aliscioni SS, Giussani LM, Kellogg EA. 2008. The age of the grasses and clusters of origins of C4 photosynthesis. Global Change Biology 14: 2963–2977. [Google Scholar]
- Vigeland MD, Spannagl M, Asp T, Paina C, Rudi H, Rognli O‐A, Fjellheim S, Sandve SR. 2013. Evidence for adaptive evolution of low‐temperature stress response genes in a Pooideae grass ancestor. New Phytologist 199: 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaverde T, Escudero M, Martín‐Bravo S, Jiménez‐Mejías P, Sanmartín I, Vargas P, Luceño M. 2017. Bipolar distributions in vascular plants: a review. American Journal of Botany 104: 1680–1694. [DOI] [PubMed] [Google Scholar]
- Wang Q, Huang J, Zang R, Li Z, El‐Kassaby YA. 2022. Centres of neo‐and paleo‐endemism for Chinese woody flora and their environmental features. Biological Conservation 276: 109817. [Google Scholar]
- Zhang C, Rabiee M, Sayyari E, Mirarab S. 2018. ASTRAL‐III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinformatics 19: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XX, Ye JF, Laffan SW, Mishler BD, Thornhill AH, Lu LM, Mao LF, Liu B, Chen YH, Lu AM et al. 2022. Spatial phylogenetics of the Chinese angiosperm flora provides insights into endemism and conservation. Journal of Integrative Plant Biology 64: 105–117. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Qian L, Spalink D, Sun L, Chen J, Sun H. 2021. Spatial phylogenetics of two topographic extremes of the Hengduan Mountains in southwestern China and its implications for biodiversity conservation. Plant Diversity 43: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Valen E, Parker BJ, Sandelin A. 2011. Systematic clustering of transcription start site landscapes. PLoS ONE 6: e23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Ancestral area reconstruction within Poales based on seven regions, obtained using the DIVA model in BioGeoBEARS.
Fig. S2 Number of species of Poales missing from the phylogenetic data set compared to the number listed in the World Checklist of Vascular Plants as of 28 February 2022, mapped per botanical region.
Fig. S3 Phylogenetic diversity of the six largest Poales families categorised into open and closed habitats.
Fig. S4 Phylogenetic endemicity mapped per botanical region for Poales and eight families with the highest number of species in the data set.
Fig. S5 Poales botanical regions grouped into three ‘floristic kingdoms’ based on phylogenetic beta‐diversity, indicated by different colours and numbers.
Notes S1 Methodological detail on sequence assembly and tree construction.
Notes S2 Justification for selecting dispersal–extinction–cladogeneis model of ancestral estimation.
Table S1 Sequences for the phylogenetic reconstruction.
Table S2 Calibrations used in the treePL (Smith & O'Meara, 2012) configuration file.
Table S3 Taxa included in this study, showing habitat scoring across the Poales.
Table S4 Comparison of six ancestral area reconstruction models based on BioGeoBEARS analyses for Poales.
Table S5 Results from corHMM ancestral state reconstructions.
Please note: Wiley is not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
The data sets analysed in this study are available as Supporting Information. The new Angiosperms353 raw reads are deposited in the European Nucleotide Archive (PRJEB35285).


