Abstract
Multiple myeloma (MM) is a hematological malignancy caused by the clonal expansion of plasma cells. The incidence of MM worldwide is increasing with greater than 140 000 people being diagnosed with MM per year. Whereas 5‐year survival after a diagnosis of MM has improved from 28% in 1975 to 56% in 2012, the disease remains essentially incurable. In this review, we summarize our current understanding of MM including its epidemiology, genetics and biology. We will also provide an overview of MM management that has led to improvements in survival, including recent changes to diagnosis and therapies. Areas of unmet need include the management of patients with high‐risk MM, those with reduced performance status and those refractory to standard therapies. Ongoing research into the biology and early detection of MM as well as the development of novel therapies, such as immunotherapies, has the potential to influence MM practice in the future.
Keywords: clinical presentation, plasma cell disease, risks factors, survival, treatment
Abbreviations
- ASCT
autologous stem cell transplantation
- CLL
chronic lymphocytic leukemia
- FLC
free light chain
- GWAS
genome‐wide association study
- Ig
immunoglobulin
- IMWG
International Myeloma Working Group
- ISS
International staging system
- MDE
myeloma defining event
- MGUS
monoclonal gammopathy of unknown significance
- MM
multiple myeloma
- OS
overall survival
- PETHEMA
Programa Espanol de Tratamientosen Hematologia
- SEER
Surveillance, Epidemiology and End Results
- SM
smoldering myeloma
- SNP
single nucleotide polymorphism
- TTP
time to progression
- VRD
bortezomib, lenalidomide, dexamethasone
- VTD
bortezomib, thalidomide, dexamethasone
1. INTRODUCTION
Multiple myeloma (MM) is an incurable hematological malignancy caused by the clonal expansion of plasma cells. The malignant plasma cells generally reside in the bone marrow and produce an abnormal antibody (M‐protein). 1 Over 140 000 cases of MM are diagnosed worldwide per year with a lifetime risk of MM in economically developed countries of 0.6% to 1%. 2 , 3 , 4
The first case reports of MM appeared in the medical literature in the 1840s. 5 , 6 , 7 In 1848, William Macintyre and Henry Bence Jones described an abnormal protein in the urine of a patient with MM and, in 1889, Otto Kahler described the archetypical clinical features. 8 , 9 Swedish scientist Arne Tiselius developed electrophoretic isolation of serum proteins in the 1930s and in 1961, another Swedish scientist, Jan Waldenström, described the pathognomonic monoclonal M‐protein. 10 Since these landmark discoveries, our understanding of the biological basis and management of MM has progressed leading to improvements in survival.
2. CLINICAL PRESENTATION, INVESTIGATIONS AND STAGING
The most common presenting signs and symptoms of MM are anemia, bone pain, renal dysfunction, fatigue, hypercalcemia, infection and weight loss. 11 Less common features include extradural spinal cord compression (due to extramedullary plasmacytoma or a bone fragment due to a vertebral body fracture), hepatomegaly, splenomegaly and hyperviscosity. 11 The majority of MM patients have a M‐protein generated by the clonal plasma cell population. Of the different types of paraprotein produced, IgG accounts for 52% of cases, IgA 21%, light chain 16% and IgD, biclonal and IgM each account for <5% of cases. Serum protein electrophoresis will identify the abnormal protein in 80% of cases and serum protein immunofixation increases the sensitivity. 11 A serum‐free light chain assay or urinary protein electrophoresis and immunofixation increase the sensitivity further, particularly as it identifies light chain‐only disease. 11 , 12 , 13 Newer techniques based on mass spectrometry are emerging which have a number of clinical and analytical advantages when compared to serum protein electrophoresis. 14 About 6.5% of MM cases are thought to be oligosecretory or nonsecretory. 11
Monoclonal gammopathy of unknown significance (MGUS) is the progenitor disease of MM, where the patient is often asymptomatic and the M‐protein is typically present at a lower concentration than in MM (Table 1). 15 , 16 The annual risk of progression of MGUS to MM is 1%. 17 The prevalence of MGUS increases with age and is detectable in 1.7% of those aged 50 to 59 years and 6% of individuals aged over 80 years. 18 Smoldering myeloma (SM) represents an intermediate clinical stage between MGUS and MM (Table 1). 19 Other diseases related to MM, termed plasma cell dyscrasias, include light chain AL amyloidosis, and plasma cell leukemia. 19 When IgM MGUS progresses to symptomatic disease, it typically results in Waldenström's macroglobulinemia (a mature B‐cell neoplasm), although rare cases of IgM MM have been reported.
TABLE 1.
Summary of diagnostic criteria for MGUS, SM and MM
| Monoclonal protein and clonal bone marrow plasma cells | Myeloma defining event (biomarker of malignancy a or end‐organ damage) | |
|---|---|---|
| MGUS | Serum monoclonal protein <30 g/L and urinary monoclonal protein <500 mg per 24 hours and clonal bone marrow plasma cells <10% | No |
| SM | Serum monoclonal protein ≥30 g/L or urinary monoclonal protein ≥500 mg per 24 hours or clonal bone marrow plasma cells 10% to 60% | No |
| MM | Clonal bone marrow plasma cells ≥10% or biopsy‐proven plasmacytoma | Yes |
Abbreviations: MGUS, monoclonal gammopathy of undetermined significance; SM smoldering myeloma; MM, multiple myeloma. FLC ratio, involved versus uninvolved serum‐free light chain ratio.
Biomarker of malignancy: ≥60% clonal bone marrow plasma cells; ≥100 FLC ratio (absolute level of the involved light chain is at least 100 mg/L); >1 lesion by magnetic resonance imaging (≥5 mm in size). End‐organ damage (due to myeloma): hypercalcemia (serum calcium >0.25 mmol/L [>1 mg/dL] higher than the upper limit of normal or >2.75 mmol/L [>11 mg/dL]); renal insufficiency (creatinine clearance <40 mL per minute or serum creatinine >177 mol/L [>2 mg/dL]); anemia (hemoglobin >20 g/L below the lowest limit of normal or hemoglobin <100 g/L); bone lesions: one or more osteolytic lesion on skeletal radiography, CT or PET/CT. If bone marrow has <10% clonal plasma cells, more than one bone lesion is required to distinguish from solitary plasmacytoma with minimal marrow involvement.
All patients with suspected MM require cross‐sectional imaging to assess for myeloma‐related bone disease and extramedullary disease. 20 , 21 Due to the higher sensitivity when compared to conventional skeletal surveys, whole‐body low‐dose CT is the current standard first‐line imaging modality when investigating SM, MM, relapse or prior to maintenance therapy in the absence of previous FDG‐avid disease. 20 , 21 , 22 PET‐CT is recommended when investigating extramedullary solitary plasmacytomas, as an alternative in suspected MM or for revaluation of previous FDG‐avid disease prior to maintenance therapy. Whole‐body diffusion‐weighted MRI is currently recommended where low‐dose CT does not demonstrate disease where MM is suspected, is inconclusive or in the reassessment of disease after treatment, although this is becoming the primary imaging modality in some centers. 20 , 21
Quantification of plasma cell infiltration is performed using morphological assessment of a bone marrow aspirate or biopsy with or without immunohistochemistry with antibodies to plasma cell associated antigens, such as CD138. 23 , 24 Whereas the quantity of plasma cells may be underestimated by flow cytometry compared to morphological assessment, the higher sensitivity of multicolor flow cytometry allows for the detection of small numbers of plasma cells, which may be missed by morphological or immunohistochemical evaluation. 25 , 26 Cytogenetic analysis by fluorescence in situ hybridization on purified clonal plasma cells should include tests for the high‐risk cytogenetic abnormalities including t(4;14), t(14;16) and del(17p). 27
Traditionally, a diagnosis of MM required the presence of end‐organ damage, which for diagnostic purposes, took the form of the CRAB criteria (C = elevated calcium, R = renal failure, A = anemia, B = bone lesions). In 2014, the International Myeloma Working Group (IMWG) revised the diagnostic criteria for MM and plasma cell dyscrasias which is summarized in Table 1. 19 One major change was the addition of myeloma defining events (MDEs) to the traditional features of end‐organ damage when making a diagnosis of active MM. The aim of this change was to identify and treat individuals with a diagnosis of SM and a >80% probability of progression to end‐organ damage within 2 years. 19 , 28 Recently, whole‐genome sequencing (WGS) has been utilized to identify individuals with MM precursor diseases with low disease burden at a high‐risk of progression. 29 Confirmation of such findings in larger studies is required along with the assessment of the risk discrimination afforded by WGS.
The factors which influence clinical outcomes of patients with MM can be divided into characteristics related to the tumor and those related to the patient. The MM International Staging System (ISS) formalizes such features and is based on serum albumin and β2‐microglobulin (β2M) concentrations. 30 This staging system has been refined as the Revised International Staging System (R‐ISS), which incorporates information concerning somatic genetics, namely t(4;14), t(14;16) and del(17p), and lactate dehydrogenase concentration (Table 2). 31 There is no unified definition of high‐risk myeloma (patients who experience early disease progression and death) but characteristics used include gene expression profiling, ISS Stage III disease, extramedullary disease or plasma cell leukemia or the presence of del(17p), 1q21 gain, t(4;14), t(14;16). 32 , 33 Additional somatic genomic classifiers such as biallelic TP53 inactivation or amplification (≥4 copies) of CKS1B (1q21) have been found to add further discrimination beyond the R‐ISS. 33 , 34
TABLE 2.
The International Myeloma Working Group revised international staging system (R‐ISS) 31
| Stage | R‐ISS | 5‐year OS |
|---|---|---|
| I |
Serum albumin >3.5 g/dL Serum β2‐microglobulin <3.5 mg/L No high‐risk cytogenetic features Normal serum lactate dehydrogenase level |
82% |
| II | Neither Stage I or III | 62% |
| III | Serum β2‐microglobulin >3.5 mg/L and high‐risk cytogenetics (t(4;14), t(14;16), del(17p)) or elevated serum lactate dehydrogenase level | 40% |
Abbreviation: OS, overall survival.
3. EPIDEMIOLOGY OF MYELOMA
The incidence of MM varies by sociodemographic status with the highest rate in high‐income countries (4‐6 per 100 000) and a 10‐fold difference between countries with the lowest and the highest rates. 4 From 1990 to 2016, the incidence of MM has increased by 126%. 4 This increase is largely due to a rise in age‐specific incidence rate, an aging population and population growth. Under‐reporting of cases at the start of cancer registries, changes to the diagnostic classification as well as resource‐stratified guidelines are likely to have contributed to the increase in the number of cases. 35 In countries with a high sociodemographic index, mortality from MM peaked in the year 2000 whereas, in most other countries, mortality from MM continues to increase. 4 Such trends can be explained by disproportionate improvements in care in regions with a high sociodemographic status. 4 , 36
Figure 1 shows the incidence and mortality rates (adjusted to world standard age structure) for Denmark. The Danish data are unique in that they originate from the first national cancer registry in the world (established in 1943). The other aspect is that Copenhagen, the capital of Denmark, is located only 30 km from the city of Malmö, where Jan Waldenström was working. Thus, it is likely that MM was a well‐known disease in Denmark. The incidence rates for men and women have increased 6‐fold in the 73‐year period through 2016 (Figure 1). The male rates are 50% higher than the female rates but the increase in both is parallel. Mortality rates are close to parallel, with two maxima, one in the early 1960s and the second around 1990. Until the early 1960s, the mortality rate from MM was higher than the incidence rate. Given death registration is independent of cancer registration, Figure 1 suggests that there was a large under‐registration of incident MM cases, which the death registrar was subsequently able to attribute to MM.
FIGURE 1.
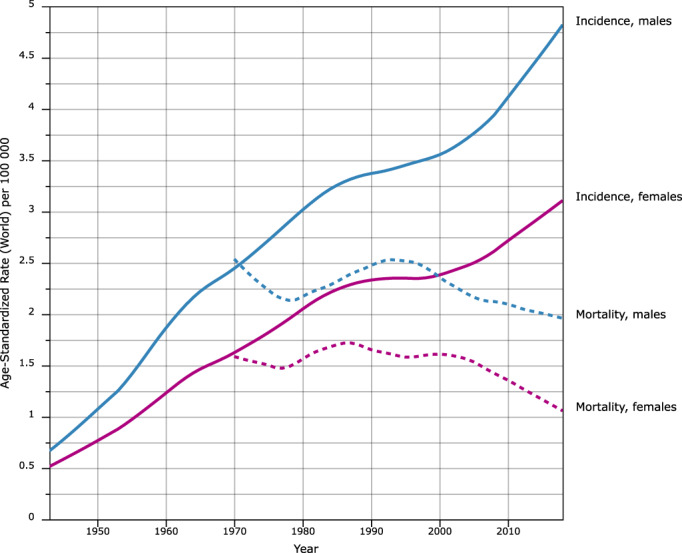
Incidence (from 1943, solid lines) and mortality (from 1950, broken lines) in multiple myeloma in Denmark to 2016. Lines corresponding to men are blue whereas lines corresponding to women are magenta. The rates are adjusted to the world standard population. The data are from the NORDCAN database from the International Agency for Research on Cancer [Color figure can be viewed at wileyonlinelibrary.com]
Under‐reporting of MM cases in Denmark becomes clearer when we review MM epidemiology in Jan Waldenström's side, Sweden (Figure S1). Whereas the sex proportions were identical to Denmark, the incidence peaked much earlier, for men before 1990 and for women in 1975; yet, the maximal incidence rates were not much different from those in Denmark 30 years later. As the death rates in the two countries were relatively parallel, it is likely that the under‐reporting in Denmark continued well into the 2000s. Even if we have no proof that our interpretation of under‐reporting is correct, the example reminds about the difficulties in interpreting observational epidemiological data between two neighboring countries, let alone on the global scale.
Changes in risk factors may provide an alternative explanation for variation in the incidence rates of MM. To date, known risk factors include ethnicity, family history and the presence of a precursor disease state (MGUS and SM). Environmental risk factors for MM and precursor diseases have been reviewed. 37 , 38 A 2.4‐fold increase in MGUS was found in US Vietnam War Veterans exposed to Agent Orange, although other confounding factors cannot be excluded as a cause for the increased risk observed. 39 The study was followed up by analysis of serum levels of microRNAs (miRNAs) in the exposed soldiers providing evidence on TCDD disrupted miRNA homeostasis. 40 Even pesticide use in agriculture has been associated with an increased risk of MGUS. 41 , 42 In an occupational health study from the Nordic countries, male and female farmers were the only population with an increased risk of MM (however relative risk of only 1.1). 43 Firefighters engaged in the containment of the World Trade Center attacks had a 2‐fold increased risk of MGUS although other confounders cannot be excluded. 44 An increased risk of MM has been reported among firefighters from the Nordic countries. 45 Risk of senile cataract and glaucoma was increased in persons earlier diagnosed with MM, MGUS, AL amyloidosis and Waldenström's macroglobulinemia. 46 The reason was suggested to be M‐protein‐related increase in blood viscosity disturbing protein structure of the lens of the eye which is exquisitely sensitive to protein aggregation; ambient protein concentration of the lens is the highest of any tissue and lens proteins are extremely long‐lived. 47 Additionally, positive associations have been described for MM and immune related factors. 48 Nevertheless, common risk factors of cancer, including cigarette smoking, obesity, socioeconomic level, educational background or radiation exposure (atomic bomb survivors) do not appear to play a role. 49 , 50 , 51 , 52 , 53 Individuals of black ethnicity have a 2‐fold increased risk of MM when compared to white individuals, whereas the incidence of MM is markedly lower in Asians. 54 Whereas there could be several explanations, it is not excluded that ethnic variations are the result of genetic differences between populations.
4. FAMILIAL RISKS
An inherited component to MM susceptibility was first suggested in the 1920s. Since then a number of families with multiple cases of MM and other plasma cell dyscrasias have been reported. The first systematic population studies emerged from Sweden in the early 2000s. According to these Swedish epidemiological studies, the familial risk of MM has been reported to be ~2.5. 55 , 56 , 57 MM is associated with an elevated familial risk of its precursor disease MGUS. 58 , 59 , 60 An increased risk has also been reported for MM with other B‐cell malignancies such as chronic lymphoid leukemia, acute lymphoblastic leukemia and lymphoplasmacytic lymphoma/Waldenstöm's macroglobulinemia as well as the myeloproliferative neoplasms. 2 , 55 , 56 , 61 Table S1 shows significant associations, as relative risks, using data from a Swedish family study on MM. 62 Significant associations with MM included colorectal, breast and prostate cancers and CLL, in addition to MM. The small excess familial risks with breast and prostate cancers may be an indication of shared risk factors between these cancers. 63
5. GERMLINE GENETICS
Motivated by epidemiological studies demonstrating familial aggregation, there has been significant interest in identifying DNA sequence variants that predispose for MM. To date, no high penetrance susceptibility loci have been identified for MM. 64 , 65 , 66 However, recent sequencing efforts have proposed novel candidates, most notably loss‐of‐function variants in DIS3 and KDM1A. 67 , 68 , 69 , 70 , 71
Support for polygenic susceptibility to MM has been provided by genome‐wide association studies (GWAS). 70 , 72 , 73 , 74 , 75 , 76 Figure S2 summarizes the most recent GWAS of MM risk comprising 9974 cases and 247 556 controls of European ancestry. The 24 loci that reach genome‐wide significance account for 16% of the SNP heritability for MM in European populations. A number of these risk loci have been subject to functional analysis, such as 7p15.3 (CDCA7L) and 5q15 (ELL2). 76 , 77 , 78 , 79 , 80 , 81 The lead SNP at 7p15.3 creates a new binding site for the transcription factor IRF4 and alters CDCA7L expression. 77 , 78 At 5q15, the risk SNPs are associated with reduced ELL2 expression. 79 , 80 ELL2 encodes a key component of the super elongation complex, which mediates rapid gene induction by suppressing transient pausing of RNA polymerase II. B cell‐lineage ELL2 conditional knockout mice exhibit diminished humoral responses to immunization, 81 and the same ELL2 allele that predisposes for MM also reduces IgA and IgG levels. 76 , 82 The arrow in Figure S2 marks the CCND1 870G>A polymorphism (11q13.3) that influences splicing of the CCND1 mRNA and is associated with translocation t(11;14) MM. This provided the first evidence for genetic variation being a determinant of a specific somatic chromosomal aberration. 83 Genome‐wide interaction and pathway‐based analysis revealed interactions with immune modulation and B‐cell development pathways. 84 A systematic analysis of MM risk loci has provided evidence of the role of disrupted cell cycle signaling, apoptosis and autophagy in MM susceptiblity 70 , 72 (Figure S3).
A genetic correlation exists between MGUS and MM suggesting that the MM risk loci exert their biological effect, at least in part, before the establishment of MGUS and contribute to familial clustering. 58 , 59 , 60 , 85 , 86 Moreover, the shared genetic risk factors observed between MM with other B‐cell malignancies such as AL amyloidosis and CLL suggests shared etiology and biology in oncogenesis. 87 , 88
6. SOMATIC GENETICS AND DISEASE BIOLOGY
The B‐cell malignancies arise from the unrestrained clonal expansion of B‐cells at different stages of maturation. 1 , 89 Conceptually, the development of MM can be thought of as an initiating transforming event occurring on the background of genetic susceptibility, the acquisition of additional somatic genetic events in the context of a microenvironment conducive to clonal expansion (Figure 2).
FIGURE 2.
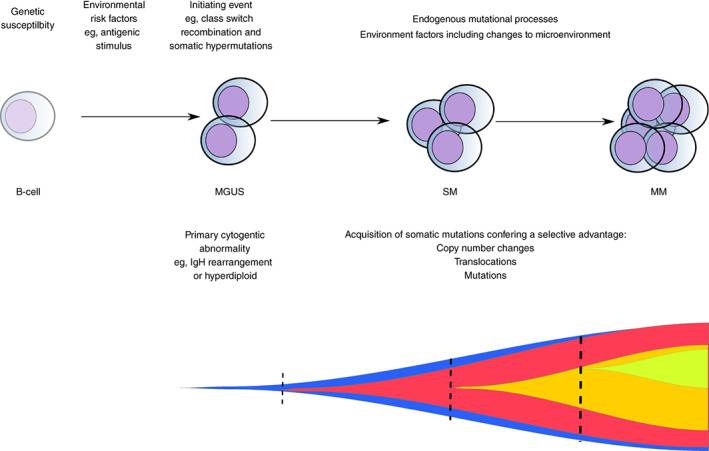
Model for the pathogenesis and evolutionary trajectory of multiple myeloma. MGUS, monoclonal gammopathy of undetermined significance; SM smoldering myeloma; MM, multiple myeloma. The fish plot demonstrates a model for the evolutionary trajectory of myeloma. The vertical dashed lines represent punctuated evolution which is characterized by the emergence of subclones that may become dominant. Static evolution occurs in‐between and represents the expansion of existing subclones under positive selection [Color figure can be viewed at wileyonlinelibrary.com]
In the germinal center, the B‐cell receptor of a naïve B‐cell undergoes class‐switch‐recombination to alter the effector function of antibodies and somatic hypermutation to increase the affinity of the B‐cell receptor to a given antigen. 90 , 91 , 92 Activation‐induced‐deaminase (AID) enzyme introduces DNA double‐strand breaks promoting both class‐switch‐recombination and somatic hypermutation. 93 Given the majority of MM cases express class‐switched immunoglobulin heavy chain (IgH) constant regions with almost all demonstrating somatic hypermutation, the clonal plasma cells which characterize MM appear to expand from a postgerminal center B‐cell. 94
The initial transforming event is thought to be an abnormal germinal center B‐cell response to an unknown antigenic stimulus. Consistent with large population‐based studies, reconstruction of the chronological activity of mutational signatures using sequencing data suggests the initial transforming event occurs in the second or third decade of life. 95 , 96
Approximately half of MGUS cases are likely caused by a primary translocation event that occurs at the time of immunoglobulin switch recombination. 97 These translocations result in the juxtaposition of the IgH locus at 14q32 with an oncogene, the most common being 11q13 (CCND1), 6p21 (CCND3), 4p16.3 (FGFT3/MMSET), 16q23 (MAF) and 20q11 (MAFB). 98 The resulting fusion causes dysregulation of the oncogene by placing it under the control of regulatory elements at the IgH locus. 99 , 100 , 101 , 102 The majority of the remaining MGUS cases are hyperdiploid, resulting in aneuploidy of chromosomes 3, 5, 7, 9, 11, 15 and 19. 103 These gains in chromosome number appear to occur early but at multiple distinct time points. 104
After the establishment of a clone with a primary cytogenetic abnormality, the acquisition of additional structural, copy number and single nucleotide variants results in a positive selection of a dominant clone as well as clonal heterogeneity. 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 Two models have been suggested to describe the evolutionary trajectory of progression. In the “static progression model”, the subclonal architecture is maintained as the disease advances suggesting progression to a clinical diagnosis reflects the time needed to accumulate a significant disease burden. In the “spontaneous evolution model”, a change in the subclonal composition is observed through the acquisition of additional mutations conferring a proliferative advantage to one of the subclones. 118 Notable copy number changes and translocations include del(1p), del(11q), del(13q), del(17p), 1q gain and translocations involving MYC. 119 , 120 , 121 , 122 Point mutations occur most often in NRAS, KRAS, BRAF, TENT5 and CDKN2C. 107 , 123 Pathways annotated by somatic genetic abnormalities include the RAS/MAPK signaling, DNA damage response and the NF‐κB pathway (Figure S3). 108 The discovery of such somatic mutations has informed the development of targeted therapies such as MEK inhibitors and BRAF inhibitors. 124 , 125 Such targeted approaches are relatively new to MM, but based on experience in other cancers, will likely be challenged by multiple resistance mechanisms as well as clonal heterogeneity. 112 , 126
As well as genetic changes, alteration of gene expression through epigenetic dysregulation, for example, the abnormal histone methylation pattern observed with MMSET overexpression in t(4;14), 127 contributes to the pathogenesis MM. Both genetic and epigenetic dysregulation converge on a number of biological processes including cell cycle perturbation and dysregulation of apoptosis. 128 , 129 , 130 , 131 , 132 , 133
In addition to the plasma cell clone, the microenvironment is reshaped in MM through induction of angiogenesis, 134 suppression of antitumor immunity, 135 , 136 and modulation of plasma cell growth by bone marrow stromal cells. 137
7. MANAGEMENT OF MGUS AND SMOLDERING MYELOMA
The annual risk of progression of MGUS to active disease is 1%. 17 For SM, the annual risk of progression to MM is 10% for the first 5 years after a diagnosis, 3% for the subsequent 5 years, and 1% thereafter. 138 However, risks vary within these groups and individuals with MGUS and SM should therefore undergo risk stratification to determine the risk of developing MM or an associated lymphoproliferative disease and to assist in determining the interval and location of follow‐up. 11 , 139 , 140 , 141 Currently used risk calculators include the Mayo clinic or PETHEMA calculator for MGUS and the PETHEMA or the revised Mayo clinic calculator (2/20/20; M‐protein >2 g/dL, bone marrow plasma cells >20%, involved/uninvolved free light chain [FLC] ratio >20) for SM. 142 , 143 , 144 Using widely available tests that are reflective of clonal plasma cell burden, the revised Mayo clinic calculator for SM identifies three risk groups (no risk factors; median time to progression [TTP] = 110 months); intermediate risk (one risk factor; TTP = 68 months); and high risk (≥2 risk factor; TTP = 29 months). The addition of cytogenetic abnormalities (MYC abnormalities, t(4;14), t(14;16), +1q and/or del13q), MAPK pathway mutations and DNA repair pathway mutations may refine risk stratification of patients with SM. 113 , 140 Treating individuals with SM at high‐risk of progression, with the aim of preventing end‐organ damage, is currently of interest. Two approaches have been advocated, a low‐intensity clonal control approach, 145 , 146 or a high‐intensity clonal eradication approach. 147 Whereas treating asymptomatic individuals with plasma cell disorders is theoretically attractive, concerns exist that early treatment adds cost and therapeutic burden in the absence of robust evidence supporting survival or a health‐related quality of life benefit. 148
Care should be taken to identify individuals with MGUS with unexplained symptoms and signs as further investigation may identify patients with monoclonal gammopathies of clinical significance. 149 Due to data demonstrating an increased risk of infection, individuals with MGUS, SM and MM in remission should be vaccinated against the influenza virus, pneumococci and hemophilus influenzae. 150 , 151 Vaccination against hepatitis A, hepatitis B, meningococcus, tetanus, diphtheria toxoids, acellular pertussis and herpes zoster is dependent on immune function, previous vaccinations and potential exposure. 151 Vaccinations should ideally occur in the absence of active disease. 152
There is significant interest in the early detection of plasma cell dyscrasias in the population. Initiatives such as the Iceland Screens Treats or Prevents Multiple Myeloma (iStopMM) study and the Promise study plan to identify individuals in the population with precursor clonal plasma cell disorders with the aim of understanding the natural history and biology of these diseases and ultimately treating individuals with high‐risk, asymptomatic disease.
8. MANAGEMENT OF MYELOMA
8.1. Historical perspective
Before the 1960s, the treatment of MM was directed toward the alleviation of symptoms rather than controlling the disease. 153 Due to the cytotoxic effects, urethane was used in the middle of the 1900s until it was shown ineffective in 1966. 154 By the late 1950s, another cytotoxic agent melphalan became available and when combined with prednisone (a steroid which in isolation reduced M‐protein levels) was the first combination therapy to produce objective response in MM. 155 , 156 With the aim of inducing complete remission through the use of larger doses of cytotoxic, high‐dose melphalan followed by autologous stem cell transplantation (ASCT) was first reported in a patient with MM at the Royal Marsden Hospital in 1983. 157 Bart Barlogie successfully used thalidomide (used initially for the anti‐angiogenic properties) in 1997 to treat a patient with MM and the first clinical trial of its use was published in 1999. 158 , 159 , 160 It was not until 2010 that CRBN was identified as a target of thalidomide and 2014 when IKZF1 and IKZF3 as the degradation targets of the CRL4CRBN E3 ubiquitin ligase. 161 Proteasome inhibitors were developed in the late 20th century with the aim of interfering with the ordered, temporal degradation of proteins responsible for cancer cell proliferation. In 2004, the first trial demonstrating the efficacy of the proteasome inhibitor Bortezomib in MM was published. 162 , 163 , 164
8.2. General principles
Such developments in antimyeloma therapies have contributed to improvements in survival. 165 , 166 , 167 Relative survival (ie, survival in MM patients compared to survival in the age‐adjusted background population) from the US Surveillance, Epidemiology and End Results (SEER) database is illustrated in Figure 3. 168 In 1975, 1‐year survival in the SEER population (all ethnics) was 68% which improved to 84% in 2016. Five‐year survival has also increased from 28% in 1975 to 56% in 2012 with survival for men and women being similar. Such improvements in survival are also seen in European countries. 167 , 169 , 170
FIGURE 3.
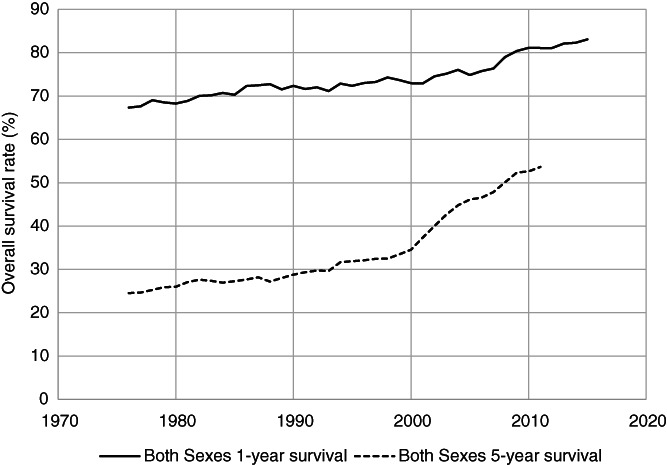
Trends in relative survival in multiple myeloma based on the US Surveillance, Epidemiology and End Results (SEER) database from 1975 through 2016, for both sexes and all ethnic groups. The upper graph is 1‐year and the lower 5‐year relative survival
At least seven different classes of agents have now been approved (Table 3). These agents are combined in doublet, triplet or quadruplet regimens, used with or without ASCT, or as continuous treatment. With such choice, defining optimal therapy at diagnosis and at each disease relapse, along with sequencing of such therapies, is challenging. Three drug regimens are most frequently used although two drug regimens have a role in certain clinical scenarios such as in frail patients. 171 , 172 , 173 , 174 , 175 Aside from allogenic stem cell transplantation, which is not routinely performed, 176 no treatment for MM is currently regarded as curative.
TABLE 3.
Classes of drugs approved for use in multiple myeloma
| Drug | Major mechanism of action | Administration route | Unwanted effects and cautions |
|---|---|---|---|
| Steroids | |||
| Prednisolone | Glucocorticoid receptor agonist | Oral | Hypertension, infection, steroid‐induced diabetes, cataracts, adrenal suppression, avascular necrosis, myopathy, mood disturbance, sleep disturbance, gastrointestinal ulcer disease |
| Dexamethasone | Glucocorticoid receptor agonist | Oral, Intravenous | Hypertension, infection, steroid‐induced diabetes, cataracts, adrenal suppression, avascular necrosis, myopathy, mood disturbance, sleep disturbance, gastrointestinal ulcer disease |
| Alkylating agents | |||
| Melphalan | Crosslinking of DNA and generation of double‐strand breaks | Oral, Intravenous | Myelosuppression, infection, mucositis, secondary malignancy, accumulation in renal failure |
| Cyclophosphamide | Crosslinking of DNA and generation of double‐strand breaks | Oral, Intravenous | Myelosuppression, infection, mucositis, secondary malignancy, hemorrhagic cystitis at high doses |
| Immunomodulatory drugs | |||
| Thalidomide | Binds to CRBN inducing proteasomal degradation of IKZF1 and IKFZ3 | Oral | Peripheral neuropathy, venous thromboembolism, somnolence, rash, teratogenic |
| Lenalidomide | Binds to CRBN inducing proteasomal degradation of IKZF1 and IKFZ3 (differing CRBN binding properties and degradation targets when compared to other immunomodulatory agents) | Oral | Myelosuppression, venous thromboembolism, diarrhea, constipation, rash, teratogenic, accumulation in renal failure, second cancers |
| Pomalidomide | Binds to CRBN inducing proteasomal degradation of IKZF1 and IKFZ3 (differing CRBN binding properties and degradation targets when compared to other immunomodulatory agents) | Oral | Bone marrow suppression, venous thromboembolism, rash, teratogenic |
| Proteasome inhibitors | |||
| Bortezomib | First‐generation reversible boronic acid proteasome inhibitor | Subcutaneous, Intravenous | Peripheral neuropathy, thrombocytopenia, gastrointestinal toxicity, herpes zoster infection |
| Carfilzomib | Second‐generation irreversible tetrapeptide epoxyketone‐based proteasome inhibitor | Intravenous | Hypertension, cardiac failure, acute renal failure; thrombotic microangiopathy; cytopenia |
| Ixazomib | Reversible boronic acid proteasome inhibitor | Oral | Thrombocytopenia, gastrointestinal toxicity, rash, lower incidence of neuropathy compared to bortezomib |
| Histone deacetylase inhibitors | |||
| Panobinostat | Pan‐deacetylase inhibitor | Oral | Thrombocytopenia, gastrointestinal toxicity |
| Nuclear export inhibitors | |||
| Selinexor | Nuclear export inhibitor | Oral | Nausea, anorexia, diarrhea, hyponatremia, thrombocytopenia, fatigue |
| Anthracyclines | |||
| Doxorubicin | Topoisomerase II inhibitor | Intravenous | Cardiac failure, myelosuppression, infection, second malignancy |
| Monoclonal antibodies | |||
| Daratumumab | Humanized CD38‐targeting antibody |
Subcutaneous, Intravenous |
Infusion‐related reactions, interference with protein electrophoresis and blood group serological testing |
| Isatuximab | Chimeric CD38‐targeting antibody | Intravenous | Infusion‐related reactions, interference with protein electrophoresis and blood group serological testing |
| Elotuzomab | Humanized SLAMF7‐targeting antibody | Intravenous | Infusion related reactions, interference with protein electrophoresis |
| Belantamab Mafodotin | Afucosylated, humanized BCMA targeting antibody conjugated to a microtubule‐disrupting drug (monomethyl auristatin F) | Intravenous | Nausea, keratopathy, thrombocytopenia |
| CAR‐T cell therapy | |||
| Idecabtagene Vicleucel (ide‐cel) | Transduction and infusion of autologous T cells with a lentiviral vector encoding a second‐generation CAR encoding an anti‐BCMA single‐chain variable fragment, a CD137 costimulatory motif and a CD3‐zeta signaling domain. | Intravenous | Cytokine release syndrome, immune effector cell‐associated toxicity, cytopenia |
Abbreviations: CAR, chimeric antigen receptors; DNA, deoxyribonucleic acid.
Motivated by higher rates of complete response seen with new treatment approaches and the use of more sensitive techniques to measure disease (minimal residual disease) such as high throughput sequencing, the IMWG have recently revised the response categories used to assess the effect of treatment. 177 Depth of response to treatment is correlated with improved patient outcomes, although this relationship is dependent on disease biology, therapy and time point of assessment. 178 , 179
After diagnosis and risk stratification, all patients should be assessed to determine eligibility for ASCT. When compared to chemotherapy alone, ASCT prolongs both progression‐free survival and overall survival and is performed immediately after induction therapy. 180 , 181 , 182 , 183 An general schema of the frontline management of MM is provided in Figure 4.
FIGURE 4.
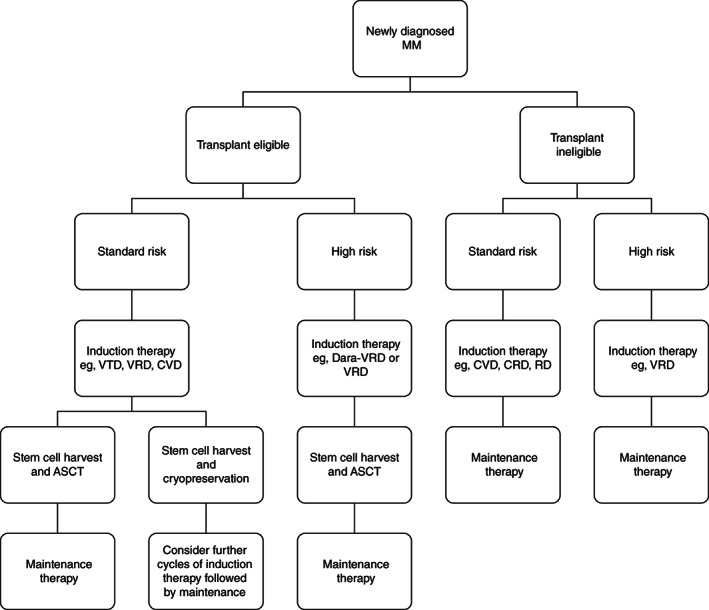
Treatment schema for newly diagnosed multiple myeloma. MM, multiple myeloma; VTD, bortezomib, thalidomide, dexamethasone; VRD, bortezomib, lenalidomide, dexamethasone; CVD, cyclophosphamide, bortezomib, dexamethasone; Dara‐VRd, daratumumab, bortezomib, lenalidomide, dexamethasone; CRD, cyclophosphamide, lenalidomide, dexamethasone; RD, lenalidomide, dexamethasone; ASCT, autologous stem cell transplantation. A guide to the current approach and possible regimens used to treat newly diagnosed multiple myeloma
8.3. Patients eligible for autologous stem cell transplantation
Induction therapy is required before ASCT to reduce disease burden, improve symptoms and mitigate organ damage. The most common treatment regimens used contain bortezomib (eg, bortezomib, thalidomide, dexamethasone [VTD] or bortezomib, lenalidomide, dexamethasone [VRD]). Overall response rates with such therapy are generally >80%. 173 , 174 , 184 , 185 After induction therapy, usually 3 to 6 cycles, a stem cell harvest is performed. These stem cells are reinfused 1 to 2 days after high‐dose chemotherapy (usually melphalan). Many centers harvest sufficient stem cells to support a second ASCT, either as a tandem procedure (for high‐risk myeloma) or at relapse. The mortality associated with ASCT is 1% to 2% and is higher in individuals with co‐morbidities such as dialysis‐dependent renal failure. 186 As the depth of response to induction therapy has improved with the use of novel therapies, the timing of ASCT has been debated. 184 , 187 , 188
The benefit of consolidation (using the same therapy at induction post‐ASCT) is not currently clear. 188 , 189 Maintenance therapy, however, in the form of single‐agent lenalidomide, has shown a survival benefit and is now routinely practiced. 190
8.4. Patients ineligible for autologous stem cell transplantation
Improvements in outcomes have been less pronounced for patients who are not eligible for ASCT. This is in part, related to poor performance status, co‐morbidities and low tolerance of multidrug regimens. 191 , 192 Improved supportive care, novel therapies and improved frailty assessment are now translating into improved outcomes in this patient population. 193 Current regimens used range from bortezomib‐based triplet therapy possibly with dose attenuation, 194 , 195 lenalidomide‐based triplet therapy, 196 and doublet therapy such lenalidomide and dexamethasone. 197
8.5. Treatment of relapsed or refractory myeloma
The majority of MM patients is relapse. This is heralded by a rise in serum M‐protein and/or light chains. Treatment should be instigated with the rapid increase in myeloma biochemical parameters or at the onset of end‐organ damage. Patients should therefore be monitored with regular assessment including measurement of myeloma biochemical parameters, and in in the absence of a biomarker, regular imaging. At relapse, the MM tumor contains significantly more mutations than the primary tumor sample. 105 Furthermore, clonal selection of mutations occur at relapse and are accompanied by subclonal heterogeneity. 105 This represents a significant therapeutic challenge.
At the time of relapse, the treatment choice is affected by patient‐related and disease‐related factors. These factors include patient preference, age, cytogenetic profile, pre‐existing toxicities, comorbidities, relapse characteristics, and by the type of, and the response to, previous therapies. 198 , 199 A change to, or the addition of, a class of drug the patient has not previously been exposed to is generally warranted at each relapse. 198 , 199 An approach to the management of MM at first relapse, along with examples of treatment regimens used is provided in Figure S4. As the number of lines of therapy increases, the time to progression and depth of response decreases. 199 The outlook is poor for patients refractory to proteasome inhibitors, immunomodulatory agents and anti‐CD38 antibodies with a median overall survival of 5.6 months. 200 For such individuals, enrolment on a clinical trial is recommended. In the absence of a clinical trial, dependent on approval and access, novel approaches such as nuclear export inhibition, 201 pan‐deacetylase inhibition, 202 an anti‐SLAMF7 antibody, 203 bispecific T‐cell engaging antibodies, 204 antibody‐drug conjugates 205 and chimeric antigen receptor T‐cell therapy are currently being used. 206 Loss of targeted antigens (such as BCMA) and soluble circulating antigens represent a challenge to targeted immunotherapies which may be overcome by γ‐secretase inhibitors and targeting multiple antigens, 207 , 208 , 209 whereas venetoclax (a BCL2 inhibitor) in combination with bortezomib in relapsed/refractory MM results in improved progression‐free survival, the development of treatment‐emergent fatal infections led to the trial closing early. 210 A subgroup analysis suggests t(11;14) MM have an improved progression‐free survival without reduced survival and response may be predicted by a high BCL2/BCL2L1 expression ratio. 211 Such findings require larger prospective trials.
8.6. Supportive care of myeloma
With an incidence of 20% to 40%, renal dysfunction is a common complication in MM and is associated with significant morbidity and mortality. 11 , 199 Renal dysfunction is multifactorial with common causes including light‐chain cast nephropathy, dehydration, hypercalcemia and less common causes such as amyloidosis and a plasma cell infiltrate. 212 , 213 Also, the optimization of renal function through treatment of infection, avoidance of dehydration and nephrotoxic drugs, antimyeloma therapy in the form of proteasome inhibitors and immunomodulatory agents improve renal function and overall survival. 214 Patients presenting acute renal failure requiring dialysis have a higher probability of renal function recovery and independence from dialysis if a rapid disease response is achieved. 215 Bortezomib‐based triplet therapy offers high rates of myeloma response and subsequent renal response. 216
Damage to the structure of bone itself is a major cause of morbidity in MM. Malignant plasma cells secrete osteoclast‐activating and osteoblast‐inhibitory factors leading to bone resorption. 217 , 218 Intravenous bisphosphonates or denosumab is initiated in patients with MM requiring therapy due to the efficacy in preventing skeletal‐related events. 219 , 220 Osteonecrosis of the jaw and atypical femoral fractures are recognized significant complications. Radiotherapy can be used to mitigate pain secondary to bone lesions and prevent fractures. 20 Surgery may be required to prevent or treat fractures as well as improve pain with significant vertebral bone disease. 221
Patients with plasma cell dyscrasia are at increased risk of thrombotic complications due to patient related factors, underlying disease and treatment. 222 , 223 Patients receiving immunomodulatory agents are particularly susceptible to thrombosis and require aspirin, low‐molecular heparin or a direct oral anticoagulant, dependent risk stratification. 224 , 225
Infection is a significant cause of morbidity and mortality in patients with MM. The highest risk in the first 3 months of induction therapy. 226 , 227 Three months of levofloxacin at induction is recommended with induction therapy. 228 Additional antimicrobial prophylaxis in the form of acyclovir, fluconazole and trimethoprim‐sulfamethoxazole is used but is treatment and center dependent. 229 Patients with MM are at significant risk of morbidity and mortality from SARS‐CoV‐2 viral infection. 230 , 231 Monitoring and treating patients with MM during the pandemic has therefore required adaptation. 232 Whereas the development and approval of vaccines will reduce transmission and risk of severe COVID‐19, there are concerns that patients with MM, particularly those with active disease, receiving treatment or with immunoparesis will not generate an appropriate antibody response. 233 , 234 , 235
9. CONCLUSION
Improvements in diagnostics, risk stratification, treatment and supportive care have led to an increase in overall survival in MM over recent decades. The increase in the number of people living with MM requires further work on the identification and management of cumulative disease and treatment‐related healthcare burdens. 236 So far, only a minority of clinical trials in MM over the past 15 years use overall survival or health‐related quality of life as a primary endpoint. 237
Despite improvements in care, MM remains incurable and the majority of patients succumb to their disease. As well as optimizing the sequencing of existing therapies, novel therapies are required particularly for patients who are refractory to approved drugs, for those with poor performance status and those with high‐risk MM (with a median OS of <2 years) from diagnosis). For example, new approaches which utilize the immune system to exert anti‐myeloma effects are becoming central to the management of MM.
Clonal heterogeneity and clonal evolution limit the prospect of genetically informed targeted therapies, in isolation, offering significant clinical benefit for a large number of patients with MM. Improving our understanding of the biological consequences of genetic susceptibility, somatic mutations and the mechanisms underlying clonal evolution represents one approach to realizing the potential of genetic studies of MM. Clonal evolution of MGUS and its progression to MM takes decades, offering a window for early intervention before the life‐threatening disease arises. MGUS is a common condition but has remained in the periphery of hematological research.
Access to drugs is currently costly which limits access in low‐income and middle‐income countries many of which have limited access to existing effective antimyeloma medications and have an increased mortality rate from MM. In high‐income countries, high‐cost drugs raise questions regarding the value of healthcare.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
Our study was supported by the European Union's Horizon 2020 Research and Innovation Program, grant No. 856620 (Chaperon). Asta Försti was supported by the German Jose Carreras Leukemia Foundation. Richard Houlston acknowledges the grant support from Cancer Research UK (C1298/A8362) and Myeloma UK. Amit Sud is in receipt of a National Institute for Health Research Academic Clinical Lectureship and funding from the Royal Marsden Biomedical Research Centre.
Hemminki K, Försti A, Houlston R, Sud A. Epidemiology, genetics and treatment of multiple myeloma and precursor diseases. Int. J. Cancer. 2021;149(12):1980‐1996. doi: 10.1002/ijc.33762
Funding information Cancer Research UK (C1298/A8362), Grant/Award Number: C1298/A8362; European Union's Horizon 2020 Research and Innovation Programme, Grant/Award Number: 856620; Royal Marsden Biomedical Research Centre; Myeloma UK; National Institute for Health
Contributor Information
Kari Hemminki, Email: k.hemminki@dkfz-heidelberg.de.
Amit Sud, Email: amit.sud@icr.ac.uk.
REFERENCES
- 1. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375‐2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sud A, Chattopadhyay S, Thomsen H, et al. Analysis of 153,115 patients with hematological malignancies refines the spectrum of familial risk. Blood. 2019;134:960‐969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smittenaar CR, Petersen KA, Stewart K, Moitt N. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer. 2016;115:1147‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cowan AJ, Allen C, Barac A, et al. Global burden of multiple myeloma: a systematic analysis for the global burden of disease study 2016. JAMA Oncol. 2018;4:1221‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kyle RA, Steensma DP. History of multiple myeloma. Recent Results Cancer Res. 2011;183:3‐23. [DOI] [PubMed] [Google Scholar]
- 6. Solly S. Remarks on the pathology of mollities ossium with cases. Med Chir Trans. 1844;27:435‐98.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rustizky JV. Multiples myelom. Deut Z Chirurg. 1873;3:162‐172. [Google Scholar]
- 8. Kahler O. Zur symptomalogii des multiple myleoms: beobachtung von albomosurie. Prag Med Wochenschr. 1889;14:45. [Google Scholar]
- 9. Jones HB III. On a new substance occurring in the urine of a patient with mollities ossium. Phil Trans R Soc Lond. 1848;138:55‐62. [Google Scholar]
- 10. Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962‐2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21‐33. [DOI] [PubMed] [Google Scholar]
- 12. Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group guidelines for serum‐free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23:215‐224. [DOI] [PubMed] [Google Scholar]
- 13. Bradwell AR, Carr‐Smith HD, Mead GP, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47:673‐680. [PubMed] [Google Scholar]
- 14. Murray DL, Puig N, Kristinsson S, et al. Mass spectrometry for the evaluation of monoclonal proteins in multiple myeloma and related disorders: an International Myeloma Working Group mass spectrometry committee report. Blood Cancer J. 2021;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Merlini G, Palladini G. Differential diagnosis of monoclonal gammopathy of undetermined significance. Hematology. 2012;2012:595‐603. [DOI] [PubMed] [Google Scholar]
- 16. van Nieuwenhuijzen N, Spaan I, Raymakers R, Peperzak V. From MGUS to multiple myeloma, a paradigm for clonal evolution of premalignant cells. Cancer Res. 2018;78:2449‐2456. [DOI] [PubMed] [Google Scholar]
- 17. Kyle RA, Larson DR, Therneau TM, et al. Long‐term follow‐up of monoclonal gammopathy of undetermined significance. N Engl J Med. 2018;378:241‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wadhera RK, Rajkumar SV. Prevalence of monoclonal gammopathy of undetermined significance: a systematic review. Mayo Clin Proc. 2010;85:933‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538‐e548. [DOI] [PubMed] [Google Scholar]
- 20. Terpos E, Zamagni E, Lentzsch S, et al. Treatment of multiple myeloma‐related bone disease: recommendations from the Bone Working Group of the International Myeloma Working Group. Lancet Oncol. 2021;22:e119‐e130. [DOI] [PubMed] [Google Scholar]
- 21. Hillengass J, Usmani S, Rajkumar SV, et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019;20:e302‐e312. [DOI] [PubMed] [Google Scholar]
- 22. Hillengass J, Moulopoulos LA, Delorme S, et al. Whole‐body computed tomography versus conventional skeletal survey in patients with multiple myeloma: a study of the International Myeloma Working Group. Blood Cancer J. 2017;7:e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bartl R, Frisch B, Burkhardt R, et al. Bone marrow histology in myeloma: its importance in diagnosis, prognosis, classification and staging. Br J Haematol. 1982;51:361‐375. [DOI] [PubMed] [Google Scholar]
- 24. Chilosi M, Adami F, Lestani M, et al. CD138/syndecan‐1: a useful immunohistochemical marker of normal and neoplastic plasma cells on routine trephine bone marrow biopsies. Mod Pathol. 1999;12:1101‐1106. [PubMed] [Google Scholar]
- 25. Morice WG, Hanson CA, Kumar S, Frederick LA, Lesnick CE, Greipp PR. Novel multi‐parameter flow cytometry sensitively detects phenotypically distinct plasma cell subsets in plasma cell proliferative disorders. Leukemia. 2007;21:2043‐2046. [DOI] [PubMed] [Google Scholar]
- 26. Rawstron AC, Orfao A, Beksac M, et al. Report of the European myeloma network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008;93:431‐438. [DOI] [PubMed] [Google Scholar]
- 27. Sonneveld P, Avet‐Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high‐risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127:2955‐2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mateos M‐V, Hernández M‐T, Giraldo P, et al. Lenalidomide plus dexamethasone for high‐risk smoldering multiple myeloma. N Engl J Med. 2013;369:438‐447. [DOI] [PubMed] [Google Scholar]
- 29. Oben B, Froyen G, Maclachlan KH, et al. Whole‐genome sequencing reveals progressive versus stable myeloma precursor conditions as two distinct entities. Nat Commun. 2021;12:1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412‐3420. [DOI] [PubMed] [Google Scholar]
- 31. Palumbo A, Avet‐Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863‐2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shah V, Sherborne AL, Johnson DC, et al. Predicting ultrahigh risk multiple myeloma by molecular profiling: an analysis of newly diagnosed transplant eligible myeloma XI trial patients. Leukemia. 2020;34:3091‐3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bolli N, Biancon G, Moarii M, et al. Analysis of the genomic landscape of multiple myeloma highlights novel prognostic markers and disease subgroups. Leukemia. 2018;32:2604‐2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walker BA, Mavrommatis K, Wardell CP, et al. A high‐risk, double‐hit, group of newly diagnosed myeloma identified by genomic analysis. Leukemia. 2019;33:159‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tan D, Chng WJ, Chou T, et al. Management of multiple myeloma in Asia: resource‐stratified guidelines. Lancet Oncol. 2013;14:e571‐e581. [DOI] [PubMed] [Google Scholar]
- 36. Keykhaei M, Masinaei M, Mohammadi E, et al. A global, regional, and national survey on burden and quality of care index (QCI) of hematologic malignancies; global burden of disease systematic analysis 1990‐2017. Exp Hematol Oncol. 2021;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kyle RA, Rajkumar SV. Epidemiology of the plasma‐cell disorders. Best Pract Res Clin Haematol. 2007;20:637‐664. [DOI] [PubMed] [Google Scholar]
- 38. Went M, Cornish AJ, Law PJ, et al. Search for multiple myeloma risk factors using Mendelian randomization. Blood Adv. 2020;4:2172‐2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Landgren O, Shim YK, Michalek J, et al. Agent Orange exposure and monoclonal Gammopathy of undetermined significance: an operation ranch hand veteran cohort study. JAMA Oncol. 2015;1:1061‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang W, Shim YK, Michalek JE, et al. Serum microRNA profiles among dioxin exposed veterans with monoclonal gammopathy of undetermined significance. J Toxicol Environ Health A. 2020;83:269‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Landgren O, Kyle RA, Hoppin JA, et al. Pesticide exposure and risk of monoclonal gammopathy of undetermined significance in the agricultural health study. Blood. 2009;113:6386‐6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hofmann JN, Beane Freeman LE, Murata K, et al. Lifetime pesticide use and monoclonal Gammopathy of undetermined significance in a prospective cohort of male farmers. Environ Health Perspect. 2021;129:17003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pukkala E, Martinsen JI, Lynge E, et al. Occupation and cancer—follow‐up of 15 million people in five Nordic countries. Acta Oncol. 2009;48:646‐790. [DOI] [PubMed] [Google Scholar]
- 44. Landgren O, Zeig‐Owens R, Giricz O, et al. Multiple myeloma and its precursor disease among firefighters exposed to the world trade center disaster. JAMA Oncol. 2018;4:821‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pukkala E, Martinsen JI, Weiderpass E, et al. Cancer incidence among firefighters: 45 years of follow‐up in five Nordic countries. Occup Environ Med. 2014;71:398‐404. [DOI] [PubMed] [Google Scholar]
- 46. Hemminki K, Försti A, Tuuminen R, et al. The incidence of senile cataract and glaucoma is increased in patients with plasma cell dyscrasias: etiologic implications. Sci Rep. 2016;6:28500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Petrash JM. Aging and age‐related diseases of the ocular lens and vitreous body. Invest Ophthalmol Vis Sci. 2013;54:Orsf54‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lindqvist EK, Goldin LR, Landgren O, et al. Personal and family history of immune‐related conditions increase the risk of plasma cell disorders: a population‐based study. Blood. 2011;118:6284‐6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hemminki K, Li X. Level of education and the risk of cancer in Sweden. Cancer Epidemiol Biomarkers Prev. 2003;12:796‐802. [PubMed] [Google Scholar]
- 50. Hemminki K, Zhang H, Czene K. Socioeconomic factors in cancer in Sweden. Int J Cancer. 2003;105:692‐700. [DOI] [PubMed] [Google Scholar]
- 51. IARC . Personal habits and indoor combustions. Vol 100E. Lyon: International Agency for Research on Cancer; 2012:575. [Google Scholar]
- 52. Went M, Sud A, Law PJ, et al. Assessing the effect of obesity‐related traits on multiple myeloma using a Mendelian randomisation approach. Blood Cancer J. 2017;7:e573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hsu WL, Preston DL, Soda M, et al. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950‐2001. Radiat Res. 2013;179:361‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marinac CR, Ghobrial IM, Birmann BM, Soiffer J, Rebbeck TR. Dissecting racial disparities in multiple myeloma. Blood Cancer J. 2020;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Altieri A, Chen B, Bermejo JL, Castro F, Hemminki K. Familial risks and temporal incidence trends of multiple myeloma. Eur J Cancer. 2006;42:1661‐1670. [DOI] [PubMed] [Google Scholar]
- 56. Landgren O, Linet MS, McMaster ML, Gridley G, Hemminki K, Goldin LR. Familial characteristics of autoimmune and hematologic disorders in 8,406 multiple myeloma patients: a population‐based case‐control study. Int J Cancer. 2006;118:3095‐3098. [DOI] [PubMed] [Google Scholar]
- 57. Hemminki K, Li X, Czene K. Familial risk of cancer: data for clinical counseling and cancer genetics. Int J Cancer. 2004;108:109‐114. [DOI] [PubMed] [Google Scholar]
- 58. Kristinsson SY, Goldin LR, Bjorkholm M, Turesson I, Landgren O. Risk of solid tumors and myeloid hematological malignancies among first‐degree relatives of patients with monoclonal gammopathy of undetermined significance. Haematologica. 2009;94:1179‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Landgren O, Kristinsson SY, Goldin LR, et al. Risk of plasma cell and lymphoproliferative disorders among 14621 first‐degree relatives of 4458 patients with monoclonal gammopathy of undetermined significance in Sweden. Blood. 2009;114:791‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vachon CM, Kyle RA, Therneau TM, et al. Increased risk of monoclonal gammopathy in first‐degree relatives of patients with multiple myeloma or monoclonal gammopathy of undetermined significance. Blood. 2009;114:785‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kristinsson SY, Bjorkholm M, Goldin LR, et al. Patterns of hematologic malignancies and solid tumors among 37,838 first‐degree relatives of 13,896 patients with multiple myeloma in Sweden. Int J Cancer. 2009;125:2147‐2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Frank C, Fallah M, Chen T, et al. Search for familial clustering of multiple myeloma with any cancer. Leukemia. 2016;30:627‐632. [DOI] [PubMed] [Google Scholar]
- 63. Frank C, Sundquist J, Yu H, Hemminki A, Hemminki K. Concordant and discordant familial cancer: familial risks, proportions and population impact. Int J Cancer. 2017;140:1510‐1516. [DOI] [PubMed] [Google Scholar]
- 64. Pui CH, Nichols KE, Yang JJ. Somatic and germline genomics in paediatric acute lymphoblastic leukaemia. Nat Rev Clin Oncol. 2019;16:227‐240. [DOI] [PubMed] [Google Scholar]
- 65. Ripperger T, Bielack SS, Borkhardt A, et al. Childhood cancer predisposition syndromes—a concise review and recommendations by the Cancer Predisposition Working Group of the Society for Pediatric Oncology and Hematology. Am J Med Genet A. 2017;173:1017‐1037. [DOI] [PubMed] [Google Scholar]
- 66. Zhang J, Walsh MF, Wu G, et al. Germline mutations in predisposition genes in pediatric Cancer. N Engl J Med. 2015;373:2336‐2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pertesi M, Vallée M, Wei X, et al. Exome sequencing identifies germline variants in DIS3 in familial multiple myeloma. Leukemia. 2019;33:2324‐2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Waller RG, Darlington TM, Wei X, et al. Novel pedigree analysis implicates DNA repair and chromatin remodeling in multiple myeloma risk. PLoS Genet. 2018;14:e1007111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wei X, Calvo‐Vidal MN, Chen S, et al. Germline lysine‐specific demethylase 1 (LSD1/KDM1A) mutations confer susceptibility to multiple myeloma. Cancer Res. 2018;78:2747‐2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pertesi M, Went M, Hansson M, Hemminki K, Houlston RS, Nilsson B. Genetic predisposition for multiple myeloma. Leukemia. 2020;34:697‐708. [DOI] [PubMed] [Google Scholar]
- 71. Catalano C, Paramasivam N, Blocka J, et al. Characterization of rare germline variants in familial multiple myeloma. Blood Cancer J. 2021;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Went M, Sud A, Forsti A, et al. Identification of multiple risk loci and regulatory mechanisms influencing susceptibility to multiple myeloma. Nat Commun. 2018;9:3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Broderick P, Chubb D, Johnson DC, et al. Common variation at 3p22.1 and 7p15.3 influences multiple myeloma risk. Nat Genet. 2012;44:58‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chubb D, Weinhold N, Broderick P, et al. Common variation at 3q26.2, 6p21.33, 17p11.2 and 22q13.1 influences multiple myeloma risk. Nat Genet. 2013;45:1221‐1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mitchell JS, Li N, Weinhold N, et al. Genome‐wide association study identifies multiple susceptibility loci for multiple myeloma. Nat Commun. 2016;7:12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Swaminathan B, Thorleifsson G, Joud M, et al. Variants in ELL2 influencing immunoglobulin levels associate with multiple myeloma. Nat Commun. 2015;6:7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li N, Johnson DC, Weinhold N, et al. Multiple myeloma risk variant at 7p15.3 creates an IRF4‐binding site and interferes with CDCA7L expression. Nat Commun. 2016;7:13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Weinhold N, Meissner T, Johnson DC, et al. The 7p15.3 (rs4487645) association for multiple myeloma shows strong allele‐specific regulation of the MYC‐interacting gene CDCA7L in malignant plasma cells. Haematologica. 2015;100:e110‐e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ali M, Ajore R, Wihlborg AK, et al. The multiple myeloma risk allele at 5q15 lowers ELL2 expression and increases ribosomal gene expression. Nat Commun. 2018;9:1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li N, Johnson DC, Weinhold N, et al. Genetic predisposition to multiple myeloma at 5q15 is mediated by an ELL2 enhancer polymorphism. Cell Rep. 2017;20:2556‐2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Park KS, Bayles I, Szlachta‐McGinn A, et al. Transcription elongation factor ELL2 drives Ig secretory‐specific mRNA production and the unfolded protein response. J Immunol. 2014;193:4663‐4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jonsson S, Sveinbjornsson G, de Lapuente Portilla AL, et al. Identification of sequence variants influencing immunoglobulin levels. Nat Genet. 2017;49:1182‐1191. [DOI] [PubMed] [Google Scholar]
- 83. Weinhold N, Johnson DC, Chubb D, et al. The CCND1 G870A polymorphism is a risk factor for t(11;14)(q13;q32) multiple myeloma. Nat Genet. 2013;45:522‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chattopadhyay S, Thomsen H, Yadav P, et al. Genome‐wide interaction and pathway‐based identification of key regulators in multiple myeloma. Commun Biol. 2019;2:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Thomsen H, Campo C, Weinhold N, et al. Genome‐wide association study on monoclonal gammopathy of unknown significance (MGUS). Eur J Haematol. 2017;99:70‐79. [DOI] [PubMed] [Google Scholar]
- 86. Clay‐Gilmour AI, Hildebrandt MAT, Brown EE, et al. Coinherited genetics of multiple myeloma and its precursor, monoclonal gammopathy of undetermined significance. Blood Adv. 2020;4:2789‐2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Went M, Sud A, Speedy H, et al. Genetic correlation between multiple myeloma and chronic lymphocytic leukaemia provides evidence for shared aetiology. Blood Cancer J. 2018;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chattopadhyay S, Thomsen H, Weinhold N, et al. Eight novel loci implicate shared genetic etiology in multiple myeloma, AL amyloidosis, and monoclonal gammopathy of unknown significance. Leukemia. 2020;34:1187‐1191. [DOI] [PubMed] [Google Scholar]
- 89. Shaffer AL, Rosenwald A, Staudt LM. Lymphoid malignancies: the dark side of B‐cell differentiation. Nat Rev Immunol. 2002;2:920‐932. [DOI] [PubMed] [Google Scholar]
- 90. Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389‐392. [DOI] [PubMed] [Google Scholar]
- 91. O'Brien RL, Brinster RL, Storb U. Somatic hypermutation of an immunoglobulin transgene in K transgenic mice. Nature. 1987;326:405‐409. [DOI] [PubMed] [Google Scholar]
- 92. Liu YJ, Malisan F, de Bouteiller O, et al. Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity. 1996;4:241‐250. [DOI] [PubMed] [Google Scholar]
- 93. Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and Hypermutation require activation‐induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553‐563. [DOI] [PubMed] [Google Scholar]
- 94. Bakkus M, Heirman C, Van Riet I, van Camp B, Thielemans K. Evidence that multiple myeloma Ig heavy chain VDJ genes contain somatic mutations but show no intraclonal variation. Blood. 1992;80:2326‐2335. [PubMed] [Google Scholar]
- 95. Rustad EH, Yellapantula V, Leongamornlert D, et al. Timing the initiation of multiple myeloma. Nat Commun. 2020;11:1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412‐5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Avet‐Loiseau H, Facon T, Grosbois B, et al. Oncogenesis of multiple myeloma: 14q32 and 13q chromosomal abnormalities are not randomly distributed, but correlate with natural history, immunological features, and clinical presentation. Blood. 2002;99:2185‐2191. [DOI] [PubMed] [Google Scholar]
- 98. Avet‐Loiseau H, Li JY, Facon T, et al. High incidence of translocations t(11;14)(q13;q32) and t(4;14)(p16;q32) in patients with plasma cell malignancies. Cancer Res. 1998;58:5640‐5645. [PubMed] [Google Scholar]
- 99. Chesi M, Bergsagel P, Brents L, Smith C, Gerhard D, Kuehl W. Dysregulation of cyclin D1 by translocation into an IgH gamma switch region in two multiple myeloma cell lines [see comments]. Blood. 1996;88:674‐681. [PubMed] [Google Scholar]
- 100. Chesi M, Nardini E, Lim RSC, Smith KD, Kuehl WM, Bergsagel PL. The t(4;14) translocation in myeloma dysregulates both FGFR3and a novel gene, MMSET, resulting in IgH/MMSET hybrid transcripts. Blood. 1998;92:3025‐3034. [PubMed] [Google Scholar]
- 101. Chesi M, Bergsagel PL, Shonukan OO, et al. Frequent dysregulation of the c‐maf proto‐oncogene at 16q23 by translocation to an Ig locus in multiple myeloma. Blood. 1998;91:4457‐4463. [PubMed] [Google Scholar]
- 102. González D, van der Burg M, García‐Sanz R, et al. Immunoglobulin gene rearrangements and the pathogenesis of multiple myeloma. Blood. 2007;110:3112‐3121. [DOI] [PubMed] [Google Scholar]
- 103. Chng WJ, van Wier SA, Ahmann GJ, et al. A validated FISH trisomy index demonstrates the hyperdiploid and nonhyperdiploid dichotomy in MGUS. Blood. 2005;106:2156‐2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Maura F, Bolli N, Angelopoulos N, et al. Genomic landscape and chronological reconstruction of driver events in multiple myeloma. Nat Commun. 2019;10:3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hoang PH, Cornish AJ, Sherborne AL, et al. An enhanced genetic model of relapsed IGH‐translocated multiple myeloma evolutionary dynamics. Blood Cancer J. 2020;10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Diamond B, Yellapantula V, Rustad EH, et al. Positive selection as the unifying force for clonal evolution in multiple myeloma. Leukemia. 2021;35:1511‐1515. [DOI] [PubMed] [Google Scholar]
- 107. Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471:467‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hoang PH, Dobbins SE, Cornish AJ, et al. Whole‐genome sequencing of multiple myeloma reveals oncogenic pathways are targeted somatically through multiple mechanisms. Leukemia. 2018;32:2459‐2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Walker BA, Wardell CP, Melchor L, et al. Intraclonal heterogeneity and distinct molecular mechanisms characterize the development of t(4;14) and t(11;14) myeloma. Blood. 2012;120:1077‐1086. [DOI] [PubMed] [Google Scholar]
- 110. Walker BA, Wardell CP, Melchor L, et al. Intraclonal heterogeneity is a critical early event in the development of myeloma and precedes the development of clinical symptoms. Leukemia. 2014;28:384‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Walker BA, Mavrommatis K, Wardell CP, et al. Identification of novel mutational drivers reveals oncogene dependencies in multiple myeloma. Blood. 2018;132:587‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bolli N, Avet‐Loiseau H, Wedge DC, et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat Commun. 2014;5:2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bustoros M, Sklavenitis‐Pistofidis R, Park J, et al. Genomic profiling of smoldering multiple myeloma identifies patients at a high risk of disease progression. J Clin Oncol. 2020;38:2380‐2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dutta AK, Fink JL, Grady JP, et al. Subclonal evolution in disease progression from MGUS/SMM to multiple myeloma is characterised by clonal stability. Leukemia. 2019;33:457‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rustad EH, Yellapantula VD, Glodzik D, et al. Revealing the impact of structural variants in multiple myeloma. Blood Cancer Discov. 2020;1:258‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Boyle EM, Deshpande S, Tytarenko R, et al. The molecular make up of smoldering myeloma highlights the evolutionary pathways leading to multiple myeloma. Nat Commun. 2021;12:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rasche L, Chavan SS, Stephens OW, et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi‐region sequencing. Nat Commun. 2017;8:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bolli N, Maura F, Minvielle S, et al. Genomic patterns of progression in smoldering multiple myeloma. Nat Commun. 2018;9:3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Shou Y, Martelli ML, Gabrea A, et al. Diverse karyotypic abnormalities of the c‐myc locus associated with c‐myc dysregulation and tumor progression in multiple myeloma. Proc Natl Acad Sci USA. 2000;97:228‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Avet‐Loiseau H, Gerson F, Magrangeas F, Minvielle S, Harousseau JL, Bataille R. Rearrangements of the c‐Myc oncogene are present in 15% of primary human multiple myeloma tumors. Blood. 2001;98:3082‐3086. [DOI] [PubMed] [Google Scholar]
- 121. Walker BA, Leone PE, Chiecchio L, et al. A compendium of myeloma‐associated chromosomal copy number abnormalities and their prognostic value. Blood. 2010;116:e56‐e65. [DOI] [PubMed] [Google Scholar]
- 122. López‐Corral L, Gutiérrez NC, Vidriales MB, et al. The progression from MGUS to smoldering myeloma and eventually to multiple myeloma involves a clonal expansion of genetically abnormal plasma cells. Clin Cancer Res. 2011;17:1692‐1700. [DOI] [PubMed] [Google Scholar]
- 123. Walker BA, Boyle EM, Wardell CP, et al. Mutational spectrum, copy number changes, and outcome: results of a sequencing study of patients with newly diagnosed myeloma. J Clin Oncol. 2015;33:3911‐3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Andrulis M, Lehners N, Capper D, et al. Targeting the BRAF V600E mutation in multiple myeloma. Cancer Discov. 2013;3:862‐869. [DOI] [PubMed] [Google Scholar]
- 125. Sriskandarajah P, De Haven BA, MacLeod K, et al. Combined targeting of MEK and the glucocorticoid receptor for the treatment of RAS‐mutant multiple myeloma. BMC Cancer. 2020;20:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Raab MS, Lehners N, Xu J, et al. Spatially divergent clonal evolution in multiple myeloma: overcoming resistance to BRAF inhibition. Blood. 2016;127:2155‐2157. [DOI] [PubMed] [Google Scholar]
- 127. Martinez‐Garcia E, Popovic R, Min D‐J, et al. The MMSET histone methyl transferase switches global histone methylation and alters gene expression in t(4;14) multiple myeloma cells. Blood. 2011;117:211‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Guillerm G, Gyan E, Wolowiec D, et al. p16(INK4a) and p15(INK4b) gene methylations in plasma cells from monoclonal gammopathy of undetermined significance. Blood. 2001;98:244‐246. [DOI] [PubMed] [Google Scholar]
- 129. Ng MH, Chung YF, Lo KW, Wickham NW, Lee JC, Huang DP. Frequent hypermethylation of p16 and p15 genes in multiple myeloma. Blood. 1997;89:2500‐2506. [PubMed] [Google Scholar]
- 130. Kulkarni MS, Daggett JL, Bender TP, Kuehl WM, Bergsagel PL, Williams ME. Frequent inactivation of the cyclin‐dependent kinase inhibitor p18 by homozygous deletion in multiple myeloma cell lines: ectopic p18 expression inhibits growth and induces apoptosis. Leukemia. 2002;16:127‐134. [DOI] [PubMed] [Google Scholar]
- 131. Derenne S, Monia B, Dean NM, et al. Antisense strategy shows that Mcl‐1 rather than Bcl‐2 or Bcl‐x(L) is an essential survival protein of human myeloma cells. Blood. 2002;100:194‐199. [DOI] [PubMed] [Google Scholar]
- 132. Ong F, van Nieuwkoop JA, de Groot‐Swings GM, et al. Bcl‐2 protein expression is not related to short survival in multiple myeloma. Leukemia. 1995;9:1282‐1284. [PubMed] [Google Scholar]
- 133. Schwarze MM, Hawley RG. Prevention of myeloma cell apoptosis by ectopic bcl‐2 expression or interleukin 6‐mediated up‐regulation of bcl‐xL. Cancer Res. 1995;55:2262‐2265. [PubMed] [Google Scholar]
- 134. Rajkumar SV, Mesa RA, Fonseca R, et al. Bone marrow angiogenesis in 400 patients with monoclonal gammopathy of undetermined significance, multiple myeloma, and primary amyloidosis. Clin Cancer Res. 2002;8:2210‐2216. [PubMed] [Google Scholar]
- 135. Zavidij O, Haradhvala NJ, Mouhieddine TH, et al. Single‐cell RNA sequencing reveals compromised immune microenvironment in precursor stages of multiple myeloma. Nat Cancer. 2020;1:493‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Liu R, Gao Q, Foltz SM, et al. Co‐evolution of tumor and immune cells during progression of multiple myeloma. Nat Commun. 2021;12:2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Arnulf B, Lecourt S, Soulier J, et al. Phenotypic and functional characterization of bone marrow mesenchymal stem cells derived from patients with multiple myeloma. Leukemia. 2007;21:158‐163. [DOI] [PubMed] [Google Scholar]
- 138. Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582‐2590. [DOI] [PubMed] [Google Scholar]
- 139. Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Mateos M‐V, Kumar S, Dimopoulos MA, et al. International Myeloma Working Group risk stratification model for smoldering multiple myeloma (SMM). Blood Cancer J. 2020;10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kyle RA, Durie BGM, Rajkumar SV, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121‐1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kyle RA, Therneau TM, Rajkumar SV, et al. A long‐term study of prognosis in monoclonal Gammopathy of undetermined significance. N Engl J Med. 2002;346:564‐569. [DOI] [PubMed] [Google Scholar]
- 143. Pérez‐Persona E, Vidriales M‐B, Mateo G, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110:2586‐2592. [DOI] [PubMed] [Google Scholar]
- 144. Lakshman A, Rajkumar SV, Buadi FK, et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J. 2018;8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Mateos M‐V, Hernández M‐T, Giraldo P, et al. Lenalidomide plus dexamethasone versus observation in patients with high‐risk smouldering multiple myeloma (QuiRedex): long‐term follow‐up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17:1127‐1136. [DOI] [PubMed] [Google Scholar]
- 146. Lonial S, Jacobus S, Fonseca R, et al. Randomized trial of Lenalidomide versus observation in smoldering multiple myeloma. J Clin Oncol. 2020;38:1126‐1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Mateos M‐V, Martinez‐Lopez J, Rodriguez Otero P, et al. Curative strategy (GEM‐CESAR) for high‐risk smoldering myeloma (SMM): carfilzomib, lenalidomide and dexamethasone (KRd) as induction followed by HDT‐ASCT, consolidation with Krd and maintenance with Rd. Blood. 2019;134:781. [Google Scholar]
- 148. Goodman AM, Kim MS, Prasad V. Persistent challenges with treating multiple myeloma early. Blood. 2021;137:456‐458. [DOI] [PubMed] [Google Scholar]
- 149. Dispenzieri A. Monoclonal gammopathies of clinical significance. Hematology. 2020;2020:380‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kristinsson SY, Tang M, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of infections: a population‐based study. Haematologica. 2012;97:854‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ludwig H, Boccadoro M, Moreau P, et al. Recommendations for vaccination in multiple myeloma: a consensus of the European Myeloma Network. Leukemia. 2021;35:31‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hartkamp A, Mulder AH, Rijkers GT, van Velzen‐Blad H, Biesma DH. Antibody responses to pneumococcal and haemophilus vaccinations in patients with B‐cell chronic lymphocytic leukaemia. Vaccine. 2001;19:1671‐1677. [DOI] [PubMed] [Google Scholar]
- 153. Kyle RA. Five decades of therapy for multiple myeloma: a paradigm for therapeutic models. Leukemia. 2005;19:910‐912. [DOI] [PubMed] [Google Scholar]
- 154. Holland JF, Hosley H, Scharlau C, et al. A controlled trial of urethane treatment in multiple myeloma. Blood. 1966;27:328‐342. [PubMed] [Google Scholar]
- 155. Bergsagel DE, Sprague CC, Austin C, Griffith KM. Evaluation of new chemotherapeutic agents in the treatment of multiple myeloma. IV. L‐Phenylalanine mustard (NSC‐8806). Cancer Chemother Rep. 1962;21:87‐99. [PubMed] [Google Scholar]
- 156. Alexanian R, Haut A, Khan AU, et al. Treatment for multiple myeloma. Combination chemotherapy with different melphalan dose regimens. JAMA. 1969;208:1680‐1685. [DOI] [PubMed] [Google Scholar]
- 157. McElwain TJ, Powles RL. High‐dose intravenous melphalan for plasma‐cell leukaemia and myeloma. Lancet. 1983;2:822‐824. [DOI] [PubMed] [Google Scholar]
- 158. Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565‐1571. [DOI] [PubMed] [Google Scholar]
- 159. Krönke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343:301‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon‐dependent destruction of Ikaros proteins. Science. 2014;343:305‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345‐1350. [DOI] [PubMed] [Google Scholar]
- 162. Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615‐2622. [PubMed] [Google Scholar]
- 163. Orlowski RZ, Eswara JR, Lafond‐Walker A, Grever MR, Orlowski M, Dang CV. Tumor growth inhibition induced in a murine model of human Burkitt's lymphoma by a proteasome inhibitor. Cancer Res. 1998;58:4342‐4348. [PubMed] [Google Scholar]
- 164. Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609‐2617. [DOI] [PubMed] [Google Scholar]
- 165. Kyle RA, Rajkumar SV. Treatment of multiple myeloma: a comprehensive review. Clin Lymphoma Myeloma. 2009;9:278‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Bergsagel PL. Where we were, where we are, where we are going: progress in multiple myeloma. Am Soc Clin Oncol Educ Book. 2014;199‐203. [DOI] [PubMed] [Google Scholar]
- 167. Kristinsson SY, Landgren O, Dickman PW, Derolf AR, Bjorkholm M. Patterns of survival in multiple myeloma: a population‐based study of patients diagnosed in Sweden from 1973 to 2003. J Clin Oncol. 2007;25:1993‐1999. [DOI] [PubMed] [Google Scholar]
- 168. Ellis L, Woods LM, Estève J, Eloranta S, Coleman MP, Rachet B. Cancer incidence, survival and mortality: explaining the concepts. Int J Cancer. 2014;135:1774‐1782. [DOI] [PubMed] [Google Scholar]
- 169. Pulte D, Jansen L, Castro FA, et al. Trends in survival of multiple myeloma patients in Germany and the United States in the first decade of the 21st century. Br J Haematol. 2015;171:189‐196. [DOI] [PubMed] [Google Scholar]
- 170. Thorsteinsdottir S, Dickman PW, Landgren O, et al. Dramatically improved survival in multiple myeloma patients in the recent decade: results from a Swedish population‐based study. Haematologica. 2018;103:e412‐e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Bridoux F, Arnulf B, Karlin L, et al. Randomized trial comparing double versus triple Bortezomib‐based regimen in patients with multiple myeloma and acute kidney injury due to cast nephropathy. J Clin Oncol. 2020;38:2647‐2657. [DOI] [PubMed] [Google Scholar]
- 172. Magarotto V, Bringhen S, Offidani M, et al. Triplet vs doublet lenalidomide‐containing regimens for the treatment of elderly patients with newly diagnosed multiple myeloma. Blood. 2016;127:1102‐1108. [DOI] [PubMed] [Google Scholar]
- 173. Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem‐cell transplant (SWOG S0777): a randomised, open‐label, phase 3 trial. The Lancet. 2017;389:519‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem‐cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376:2075‐2085. [DOI] [PubMed] [Google Scholar]
- 175. Moreau P, Avet‐Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced‐dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118:5752‐5758. [DOI] [PubMed] [Google Scholar]
- 176. Lokhorst H, Einsele H, Vesole D, et al. International Myeloma Working Group consensus statement regarding the current status of allogeneic stem‐cell transplantation for multiple myeloma. J Clin Oncol. 2010;28:4521‐4530. [DOI] [PubMed] [Google Scholar]
- 177. Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328‐e346. [DOI] [PubMed] [Google Scholar]
- 178. Schinke C, Hoering A, Wang H, et al. The prognostic value of the depth of response in multiple myeloma depends on the time of assessment, risk status and molecular subtype. Haematologica. 2017;102:e313‐e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Munshi NC, Avet‐Loiseau H, Anderson KC, et al. A large meta‐analysis establishes the role of MRD negativity in long‐term survival outcomes in patients with multiple myeloma. Blood Adv. 2020;4:5988‐5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Attal M, Harousseau J‐L, Stoppa A‐M, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med. 1996;335:91‐97. [DOI] [PubMed] [Google Scholar]
- 181. Child JA, Morgan GJ, Davies FE, et al. High‐dose chemotherapy with hematopoietic stem‐cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875‐1883. [DOI] [PubMed] [Google Scholar]
- 182. Palumbo A, Bringhen S, Petrucci MT, et al. Intermediate‐dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood. 2004;104:3052‐3057. [DOI] [PubMed] [Google Scholar]
- 183. Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371:895‐905. [DOI] [PubMed] [Google Scholar]
- 184. Attal M, Lauwers‐Cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311‐1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Moreau P, Hulin C, Macro M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013‐04 trial. Blood. 2016;127:2569‐2574. [DOI] [PubMed] [Google Scholar]
- 186. St Bernard R, Chodirker L, Masih‐Khan E, et al. Efficacy, toxicity and mortality of autologous SCT in multiple myeloma patients with dialysis‐dependent renal failure. Bone Marrow Transplant. 2015;50:95‐99. [DOI] [PubMed] [Google Scholar]
- 187. Joseph NS, Kaufman JL, Dhodapkar MV, et al. Long‐term follow‐up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk‐adapted maintenance approach in newly diagnosed multiple myeloma. J Clin Oncol. 2020;38:1928‐1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Cavo M, Gay F, Beksac M, et al. Autologous haematopoietic stem‐cell transplantation versus bortezomib‐melphalan‐prednisone, with or without bortezomib‐lenalidomide‐dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open‐label, phase 3 study. Lancet Haematol. 2020;7:e456‐e468. [DOI] [PubMed] [Google Scholar]
- 189. Stadtmauer EA, Pasquini MC, Blackwell B, et al. Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma: results of the BMT CTN 0702 trial. J Clin Oncol. 2019;37:589‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Jackson GH, Davies FE, Pawlyn C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (myeloma XI): a multicentre, open‐label, randomised, phase 3 trial. Lancet Oncol. 2019;20:57‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Pawlyn C, Cairns D, Kaiser M, et al. The relative importance of factors predicting outcome for myeloma patients at different ages: results from 3894 patients in the myeloma XI trial. Leukemia. 2020;34:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Turesson I, Bjorkholm M, Blimark CH, Kristinsson S, Velez R, Landgren O. Rapidly changing myeloma epidemiology in the general population: increased incidence, older patients, and longer survival. Eur J Haematol. 2018;101:237‐244. 10.1111/ejh.13083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Palumbo A, Bringhen S, Mateos M‐V, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068‐2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. O'Donnell EK, Laubach JP, Yee AJ, et al. Updated results of a phase 2 study of modified Lenalidomide, Bortezomib, and dexamethasone (RVd‐lite) in transplant‐ineligible multiple myeloma. Blood. 2019;134:3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Mateos MV, Cavo M, Blade J, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open‐label, phase 3 trial. Lancet. 2020;395:132‐141. [DOI] [PubMed] [Google Scholar]
- 196. Facon T, Kumar S, Plesner T, et al. Daratumumab plus Lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380:2104‐2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant‐ineligible patients with myeloma. N Engl J Med. 2014;371:906‐917. [DOI] [PubMed] [Google Scholar]
- 198. Moreau P, Kumar SK, San Miguel J, et al. Treatment of relapsed and refractory multiple myeloma: recommendations from the International Myeloma Working Group. Lancet Oncol. 2021;22:e105‐e118. [DOI] [PubMed] [Google Scholar]
- 199. Yong K, Delforge M, Driessen C, et al. Multiple myeloma: patient outcomes in real‐world practice. Br J Haematol. 2016;175:252‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38‐targeted monoclonal antibody therapy. Leukemia. 2019;33:2266‐2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Grosicki S, Simonova M, Spicka I, et al. Once‐per‐week selinexor, bortezomib, and dexamethasone versus twice‐per‐week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open‐label, phase 3 trial. The Lancet. 2020;396:1563‐1573. [DOI] [PubMed] [Google Scholar]
- 202. Laubach JP, Schjesvold F, Mariz M, et al. Efficacy and safety of oral panobinostat plus subcutaneous bortezomib and oral dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma (PANORAMA 3): an open‐label, randomised, phase 2 study. Lancet Oncol. 2021;22:142‐154. [DOI] [PubMed] [Google Scholar]
- 203. Dimopoulos MA, Dytfeld D, Grosicki S, et al. Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N Engl J Med. 2018;379:1811‐1822. [DOI] [PubMed] [Google Scholar]
- 204. Pillarisetti K, Powers G, Luistro L, et al. Teclistamab is an active T cell‐redirecting bispecific antibody against B‐cell maturation antigen for multiple myeloma. Blood Adv. 2020;4:4538‐4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM‐2): a two‐arm, randomised, open‐label, phase 2 study. Lancet Oncol. 2020;21:207‐221. [DOI] [PubMed] [Google Scholar]
- 206. Munshi NC, Anderson LD, Shah N, et al. Idecabtagene Vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384:705‐716. [DOI] [PubMed] [Google Scholar]
- 207. Samur MK, Fulciniti M, Aktas Samur A, et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat Commun. 2021;12:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Da Vià MC, Dietrich O, Truger M, et al. Homozygous BCMA gene deletion in response to anti‐BCMA CAR T cells in a patient with multiple myeloma. Nat Med. 2021;27:616‐619. [DOI] [PubMed] [Google Scholar]
- 209. Chen H, Li M, Xu N, et al. Serum B‐cell maturation antigen (BCMA) reduces binding of anti‐BCMA antibody to multiple myeloma cells. Leuk Res. 2019;81:62‐66. [DOI] [PubMed] [Google Scholar]
- 210. Kumar SK, Harrison SJ, Cavo M, et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): a randomised, double‐blind, multicentre, phase 3 trial. Lancet Oncol. 2020;21:1630‐1642. [DOI] [PubMed] [Google Scholar]
- 211. Cleynen A, Samur M, Perrot A, et al. Variable BCL2/BCL2L1 ratio in multiple myeloma with t(11;14). Blood. 2018;132:2778‐2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Huang ZQ, Sanders PW. Localization of a single binding site for immunoglobulin light chains on human Tamm‐Horsfall glycoprotein. J Clin Invest. 1997;99:732‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Solomon A, Weiss DT, Kattine AA. Nephrotoxic potential of Bence Jones proteins. N Engl J Med. 1991;324:1845‐1851. [DOI] [PubMed] [Google Scholar]
- 214. Gonsalves WI, Leung N, Rajkumar SV, et al. Improvement in renal function and its impact on survival in patients with newly diagnosed multiple myeloma. Blood Cancer J. 2015;5:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Outcomes of newly diagnosed myeloma patients requiring dialysis: renal recovery, importance of rapid response and survival benefit. Blood Cancer J. 2017;7:e571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Dimopoulos MA, Sonneveld P, Leung N, et al. International Myeloma Working Group recommendations for the diagnosis and management of myeloma‐related renal impairment. J Clin Oncol. 2016;34:1544‐1557. [DOI] [PubMed] [Google Scholar]
- 217. Heider U, Langelotz C, Jakob C, et al. Expression of receptor activator of nuclear factor κB ligand on bone marrow plasma cells correlates with osteolytic bone disease in patients with multiple myeloma. Clin Cancer Res. 2003;9:1436‐1440. [PubMed] [Google Scholar]
- 218. Tian E, Zhan F, Walker R, et al. The role of the Wnt‐signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483‐2494. [DOI] [PubMed] [Google Scholar]
- 219. Mhaskar R, Kumar A, Miladinovic B, Djulbegovic B. Bisphosphonates in multiple myeloma: an updated network meta‐analysis. Cochrane Database Syst Rev. 2017;12:CD003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Raje N, Terpos E, Willenbacher W, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double‐blind, double‐dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19:370‐381. [DOI] [PubMed] [Google Scholar]
- 221. Hussein MA, Vrionis FD, Allison R, et al. The role of vertebral augmentation in multiple myeloma: International Myeloma Working Group Consensus Statement. Leukemia. 2008;22:1479‐1484. [DOI] [PubMed] [Google Scholar]
- 222. Kristinsson SY, Pfeiffer RM, Bjorkholm M, et al. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: a population‐based study. Blood. 2010;115:4991‐4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Kristinsson SY, Fears TR, Gridley G, et al. Deep vein thrombosis after monoclonal gammopathy of undetermined significance and multiple myeloma. Blood. 2008;112:3582‐3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide‐ and lenalidomide‐associated thrombosis in myeloma. Leukemia. 2008;22:414‐423. [DOI] [PubMed] [Google Scholar]
- 225. Man L, Morris A, Brown J, Palkimas S, Davidson K. Use of direct oral anticoagulants in patients on immunomodulatory agents. J Thromb Thrombolysis. 2017;44:298‐302. [DOI] [PubMed] [Google Scholar]
- 226. Blimark C, Holmberg E, Mellqvist UH, et al. Multiple myeloma and infections: a population‐based study on 9253 multiple myeloma patients. Haematologica. 2015;100:107‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002—Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23:9219‐9226. [DOI] [PubMed] [Google Scholar]
- 228. Drayson MT, Bowcock S, Planche T, et al. Levofloxacin prophylaxis in patients with newly diagnosed myeloma (TEAMM): a multicentre, double‐blind, placebo‐controlled, randomised, phase 3 trial. Lancet Oncol. 2019;20:1760‐1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Ludwig H, Miguel JS, Dimopoulos MA, et al. International Myeloma Working Group recommendations for global myeloma care. Leukemia. 2014;28:981‐992. [DOI] [PubMed] [Google Scholar]
- 230. Cook G, John Ashcroft A, Pratt G, et al. Real‐world assessment of the clinical impact of symptomatic infection with severe acute respiratory syndrome coronavirus (COVID‐19 disease) in patients with multiple myeloma receiving systemic anti‐cancer therapy. Br J Haematol. 2020;190:e83‐e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Chari A, Samur MK, Martinez‐Lopez J, et al. Clinical features associated with COVID‐19 outcome in multiple myeloma: first results from the International Myeloma Society data set. Blood. 2020;136:3033‐3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Malard F, Mohty M. Management of patients with multiple myeloma during the COVID‐19 pandemic. Lancet Haematol. 2020;7:e435‐e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. 2021;397:99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Bird S, Panopoulou K, Shea S, et al. Response to first vaccination against SARS‐CoV‐2 in patients with multiple myeloma. Lancet Haematol. 2021;8:e389‐e392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Chattopadhyay S, Yu H, Sud A, et al. Multiple myeloma: family history and mortality in second primary cancers. Blood Cancer J. 2018;8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Mohyuddin GR, Koehn K, Abdallah A‐O, et al. Use of endpoints in multiple myeloma randomized controlled trials over the last 15 years: a systematic review. Am J Hematol. 2021;96:690‐697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information


