Abstract
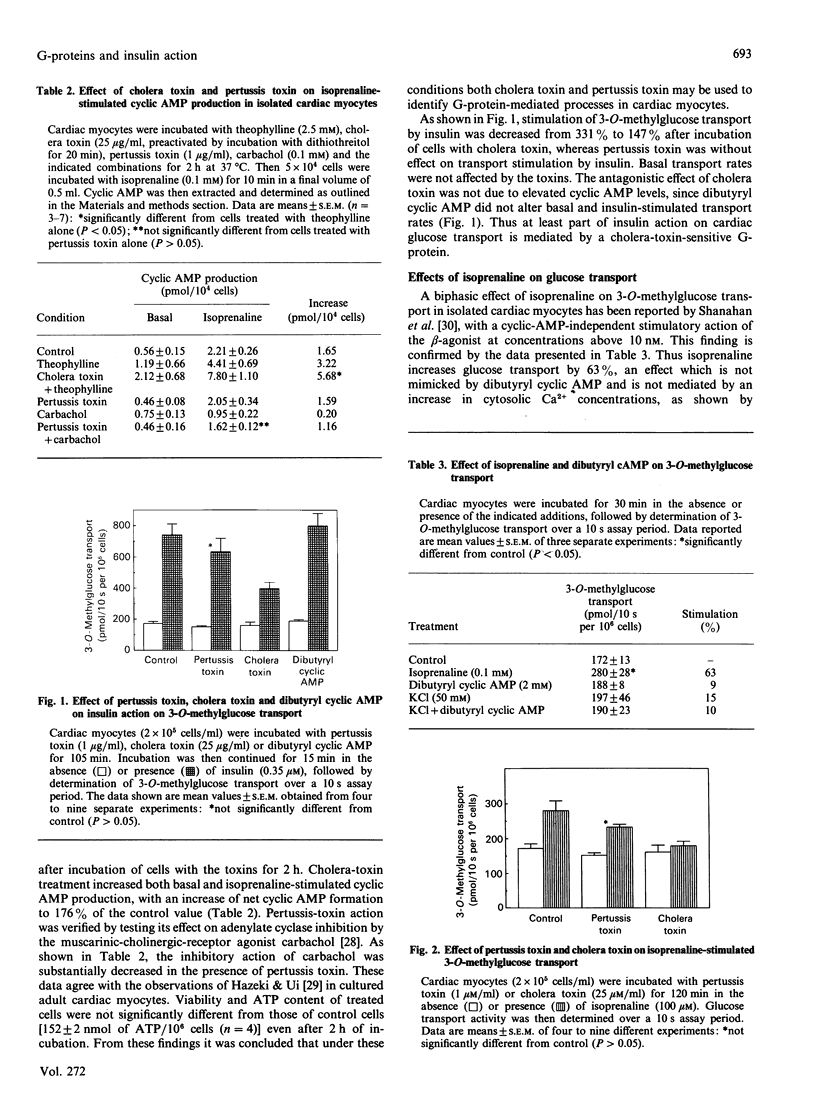
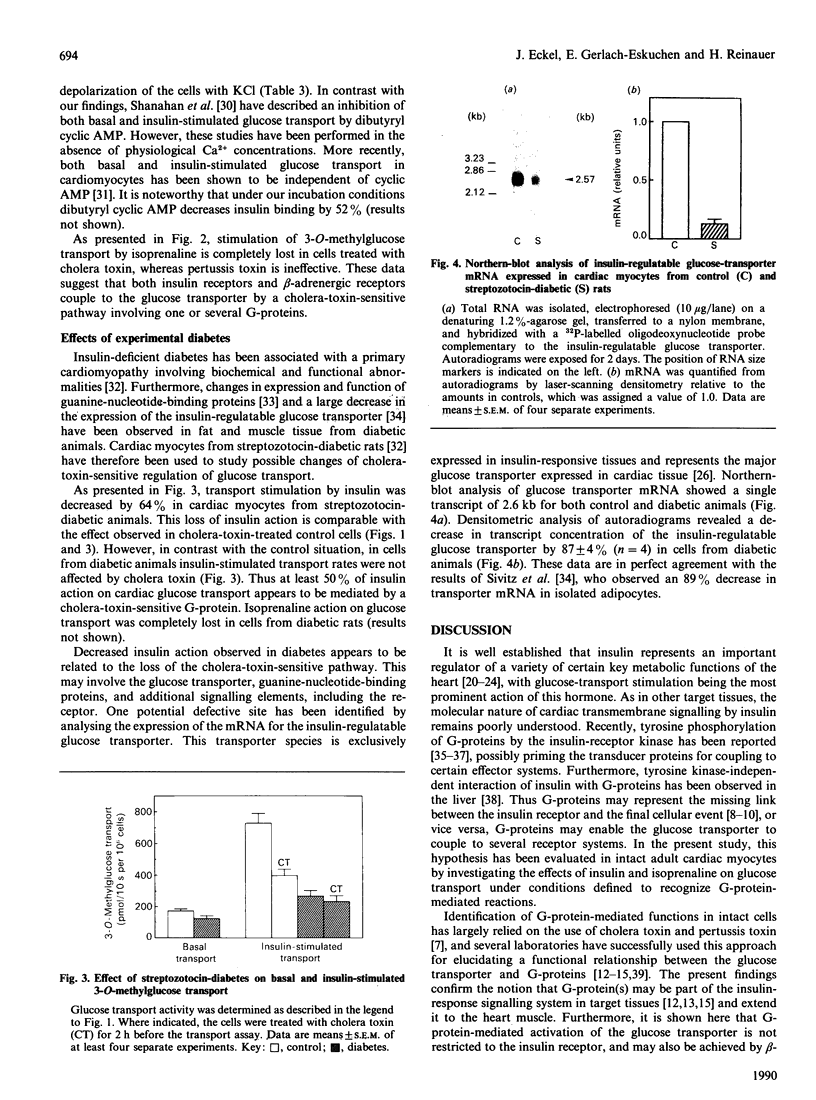
Isolated muscle cells from adult rat heart were used to study the involvement of G-proteins in the regulation of the glucose transporter by insulin and isoprenaline. Efficient modification of G-protein functions was established by measuring isoprenaline-stimulated cyclic AMP production, viability and ATP content after treating the cells with cholera toxin and pertussis toxin for 2 h. Under these conditions cholera toxin decreased the stimulatory action of insulin on 3-O-methylglucose transport by 56%, but pertussis toxin had no effect. Basal transport was not affected by toxin treatment. Isoprenaline increased 3-O-methylglucose transport by 63%. This effect was not mimicked by dibutyryl cyclic AMP, but was completely blocked by cholera toxin. Streptozotocin-diabetes abolished isoprenaline action and decreased stimulation of transport by 64%. Concomitantly, cholera-toxin sensitivity of glucose transport was lost in cells from diabetic animals. This was paralleled by a large decrease (87 +/- 4%) in mRNA expression of the insulin-regulatable glucose transporter, as shown by Northern-blot analysis of RNA isolated from cardiomyocytes of diabetic rats. These data suggest a functional association between the insulin-responsive glucose transporter and a cholera-toxin-sensitive G-protein mediating stimulation by insulin and isoprenaline.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burdett E., Mills G. B., Klip A. Effect of GTP gamma S on insulin binding and tyrosine phosphorylation in liver membranes and L6 muscle cells. Am J Physiol. 1990 Jan;258(1 Pt 1):C99–108. doi: 10.1152/ajpcell.1990.258.1.C99. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Ciaraldi T. P., Maisel A. Role of guanine nucleotide regulatory proteins in insulin stimulation of glucose transport in rat adipocytes. Influence of bacterial toxins. Biochem J. 1989 Dec 1;264(2):389–396. doi: 10.1042/bj2640389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel J., Pandalis G., Reinauer H. Insulin action on the glucose transport system in isolated cardiocytes from adult rat. Biochem J. 1983 May 15;212(2):385–392. doi: 10.1042/bj2120385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel J., Reinauer H. Effect of EDTA on insulin binding and insulin action in isolated cardiocytes from adult rat. Evidence for a functional role of low-affinity insulin receptors. Diabetes. 1984 Mar;33(3):214–218. doi: 10.2337/diab.33.3.214. [DOI] [PubMed] [Google Scholar]
- Eckel J., Reinauer H. Involvement of hormone processing in insulin-activated glucose transport by isolated cardiac myocytes. Biochem J. 1988 Jan 1;249(1):111–116. doi: 10.1042/bj2490111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel J., Reinauer H. Modulation of transmembrane potential of isolated cardiac myocytes by insulin and isoproterenol. Am J Physiol. 1990 Aug;259(2 Pt 2):H554–H559. doi: 10.1152/ajpheart.1990.259.2.H554. [DOI] [PubMed] [Google Scholar]
- Eckel J., Reinauer H. The fate of insulin in cardiac muscle. Studies on isolated muscle cells from adult rat heart. Biochem J. 1982 Sep 15;206(3):655–662. doi: 10.1042/bj2060655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel J., Röhn G., Kiesel U., Reinauer H. Insulin binding and action in isolated cardiocytes from spontaneously diabetic BB rats. Diabetes Res. 1987 Feb;4(2):79–83. [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Forsayeth J. R., Caro J. F., Sinha M. K., Maddux B. A., Goldfine I. D. Monoclonal antibodies to the human insulin receptor that activate glucose transport but not insulin receptor kinase activity. Proc Natl Acad Sci U S A. 1987 May;84(10):3448–3451. doi: 10.1073/pnas.84.10.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey W. T., Huecksteadt T. P., Birnbaum M. J. Pretranslational suppression of an insulin-responsive glucose transporter in rats with diabetes mellitus. Science. 1989 Jul 7;245(4913):60–63. doi: 10.1126/science.2662408. [DOI] [PubMed] [Google Scholar]
- Gawler D., Milligan G., Spiegel A. M., Unson C. G., Houslay M. D. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987 May 21;327(6119):229–232. doi: 10.1038/327229a0. [DOI] [PubMed] [Google Scholar]
- Geisbuhler T. P., Sergeant S., Miramonti F. L., Kim H. D., Rovetto M. J. Forskolin inhibition of hexose transport in cardiomyocytes. Pflugers Arch. 1987 Jun;409(1-2):158–162. doi: 10.1007/BF00584765. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hazeki O., Ui M. Modification by islet-activating protein of receptor-mediated regulation of cyclic AMP accumulation in isolated rat heart cells. J Biol Chem. 1981 Mar 25;256(6):2856–2862. [PubMed] [Google Scholar]
- Heyworth C. M., Houslay M. D. Insulin exerts actions through a distinct species of guanine nucleotide regulatory protein: inhibition of adenylate cyclase. Biochem J. 1983 Aug 15;214(2):547–552. doi: 10.1042/bj2140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Wallace A. V., Houslay M. D. Insulin and glucagon regulate the activation of two distinct membrane-bound cyclic AMP phosphodiesterases in hepatocytes. Biochem J. 1983 Jul 15;214(1):99–110. doi: 10.1042/bj2140099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Whetton A. D., Wong S., Martin B. R., Houslay M. D. Insulin inhibits the cholera-toxin-catalysed ribosylation of a Mr-25000 protein in rat liver plasma membranes. Biochem J. 1985 Jun 15;228(3):593–603. doi: 10.1042/bj2280593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joost H. G., Göke R. Effects of islet-activating protein on insulin- and isoprenaline-stimulated glucose transport in isolated rat adipocytes. FEBS Lett. 1984 Feb 13;167(1):5–9. doi: 10.1016/0014-5793(84)80821-2. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Göke R., Schmitz-Salue C., Steinfelder H. J., Brandenburg D. Quantitative dissociation of glucose transport stimulation and insulin receptor tyrosine kinase activation in isolated adipocytes with a covalent insulin dimer (B29,B29'-suberoyl-insulin). Biochem Pharmacol. 1989 Jul 15;38(14):2269–2277. doi: 10.1016/0006-2952(89)90465-6. [DOI] [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., Lane M. D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3150–3154. doi: 10.1073/pnas.86.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J., Rajaram R., Lakonishok M., Benovic J. L., Cerione R. A. Insulin-dependent phosphorylation of GTP-binding proteins in phospholipid vesicles. J Biol Chem. 1988 Sep 5;263(25):12333–12341. [PubMed] [Google Scholar]
- Kuroda M., Honnor R. C., Cushman S. W., Londos C., Simpson I. A. Regulation of insulin-stimulated glucose transport in the isolated rat adipocyte. cAMP-independent effects of lipolytic and antilipolytic agents. J Biol Chem. 1987 Jan 5;262(1):245–253. [PubMed] [Google Scholar]
- Luttrell L. M., Hewlett E. L., Romero G., Rogol A. D. Pertussis toxin treatment attenuates some effects of insulin in BC3H-1 murine myocytes. J Biol Chem. 1988 May 5;263(13):6134–6141. [PubMed] [Google Scholar]
- Matthaei S., Garvey W. T., Horuk R., Hueckstaedt T. P., Olefsky J. M. Human adipocyte glucose transport system. Biochemical and functional heterogeneity of hexose carriers. J Clin Invest. 1987 Mar;79(3):703–709. doi: 10.1172/JCI112874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. Techniques used in the identification and analysis of function of pertussis toxin-sensitive guanine nucleotide binding proteins. Biochem J. 1988 Oct 1;255(1):1–13. doi: 10.1042/bj2550001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T., Yasuda H. Rat heart cell membranes contain three substrates for cholera toxin-catalyzed ADP-ribosylation and a single substrate for pertussis toxin-catalyzed ADP-ribosylation. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1355–1361. doi: 10.1016/s0006-291x(86)80432-6. [DOI] [PubMed] [Google Scholar]
- O'Brien R. M., Houslay M. D., Milligan G., Siddle K. The insulin receptor tyrosyl kinase phosphorylates holomeric forms of the guanine nucleotide regulatory proteins Gi and Go. FEBS Lett. 1987 Feb 23;212(2):281–288. doi: 10.1016/0014-5793(87)81361-3. [DOI] [PubMed] [Google Scholar]
- Obermaier-Kusser B., Mühlbacher C., Mushack J., Rattenhuber E., Fehlmann M., Haring H. U. Regulation of glucose carrier activity by AlCl3 and phospholipase C in fat-cells. Biochem J. 1988 Dec 1;256(2):515–520. doi: 10.1042/bj2560515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransnäs L., Gjörstrup P., Hjalmarson A., Sjögren C. G., Jacobsson B. Muscarinic receptors in mammalian myocardium: effects of atrial and ventricular receptors on phosphatidylinositol metabolism and adenylate cyclase. J Mol Cell Cardiol. 1986 Aug;18(8):807–814. doi: 10.1016/s0022-2828(86)80955-5. [DOI] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Rothenberg P. L., Kahn C. R. Insulin inhibits pertussis toxin-catalyzed ADP-ribosylation of G-proteins. Evidence for a novel interaction between insulin receptors and G-proteins. J Biol Chem. 1988 Oct 25;263(30):15546–15552. [PubMed] [Google Scholar]
- Saltiel A. R., Fox J. A., Sherline P., Cuatrecasas P. Insulin-stimulated hydrolysis of a novel glycolipid generates modulators of cAMP phosphodiesterase. Science. 1986 Aug 29;233(4767):967–972. doi: 10.1126/science.3016898. [DOI] [PubMed] [Google Scholar]
- Schubert B., VanDongen A. M., Kirsch G. E., Brown A. M. Beta-adrenergic inhibition of cardiac sodium channels by dual G-protein pathways. Science. 1989 Aug 4;245(4917):516–519. doi: 10.1126/science.2547248. [DOI] [PubMed] [Google Scholar]
- Schürmann A., Rosenthal W., Hinsch K. D., Joost H. G. Differential sensitivity to guanine nucleotides of basal and insulin-stimulated glucose transporter activity reconstituted from adipocyte membrane fractions. FEBS Lett. 1989 Sep 25;255(2):259–264. doi: 10.1016/0014-5793(89)81102-0. [DOI] [PubMed] [Google Scholar]
- Shanahan M. F., Edwards B. M., Ruoho A. E. Interactions of insulin, catecholamines and adenosine in the regulation of glucose transport in isolated rat cardiac myocytes. Biochim Biophys Acta. 1986 Jun 16;887(1):121–129. doi: 10.1016/0167-4889(86)90132-1. [DOI] [PubMed] [Google Scholar]
- Sivitz W. I., DeSautel S. L., Kayano T., Bell G. I., Pessin J. E. Regulation of glucose transporter messenger RNA in insulin-deficient states. Nature. 1989 Jul 6;340(6228):72–74. doi: 10.1038/340072a0. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]
- Zick Y., Sagi-Eisenberg R., Pines M., Gierschik P., Spiegel A. M. Multisite phosphorylation of the alpha subunit of transducin by the insulin receptor kinase and protein kinase C. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9294–9297. doi: 10.1073/pnas.83.24.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]



