Abstract
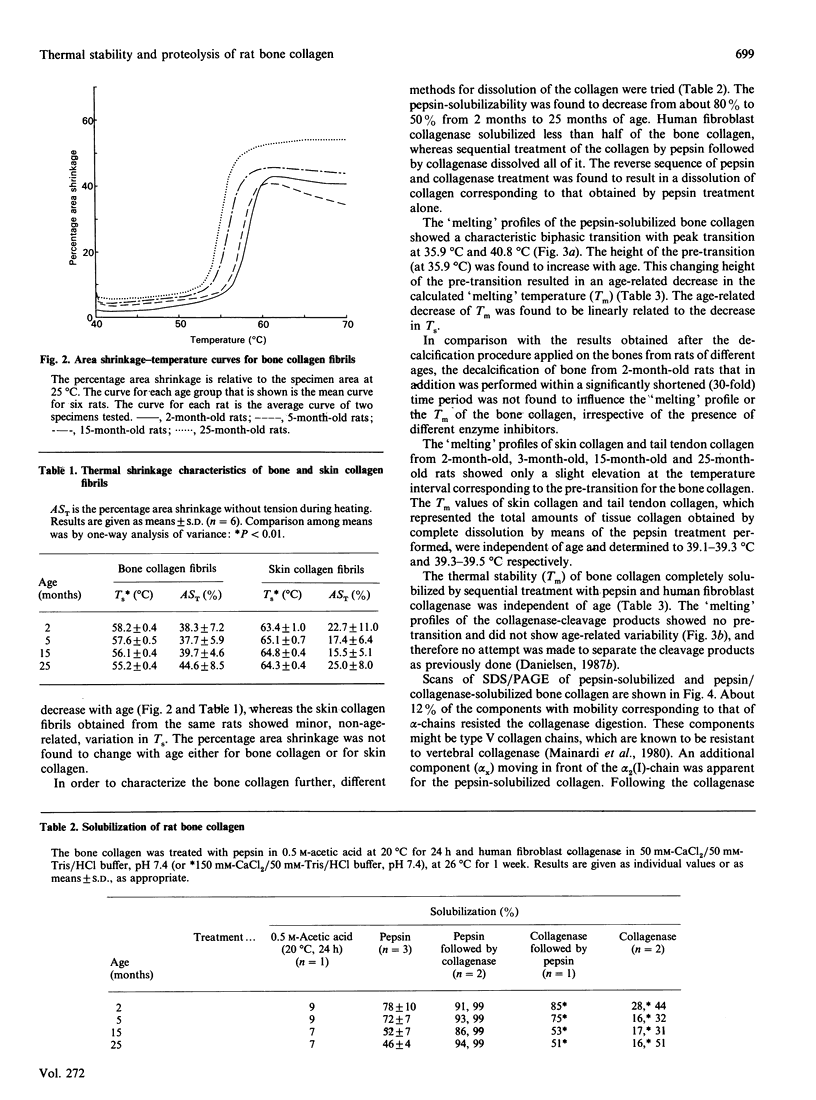
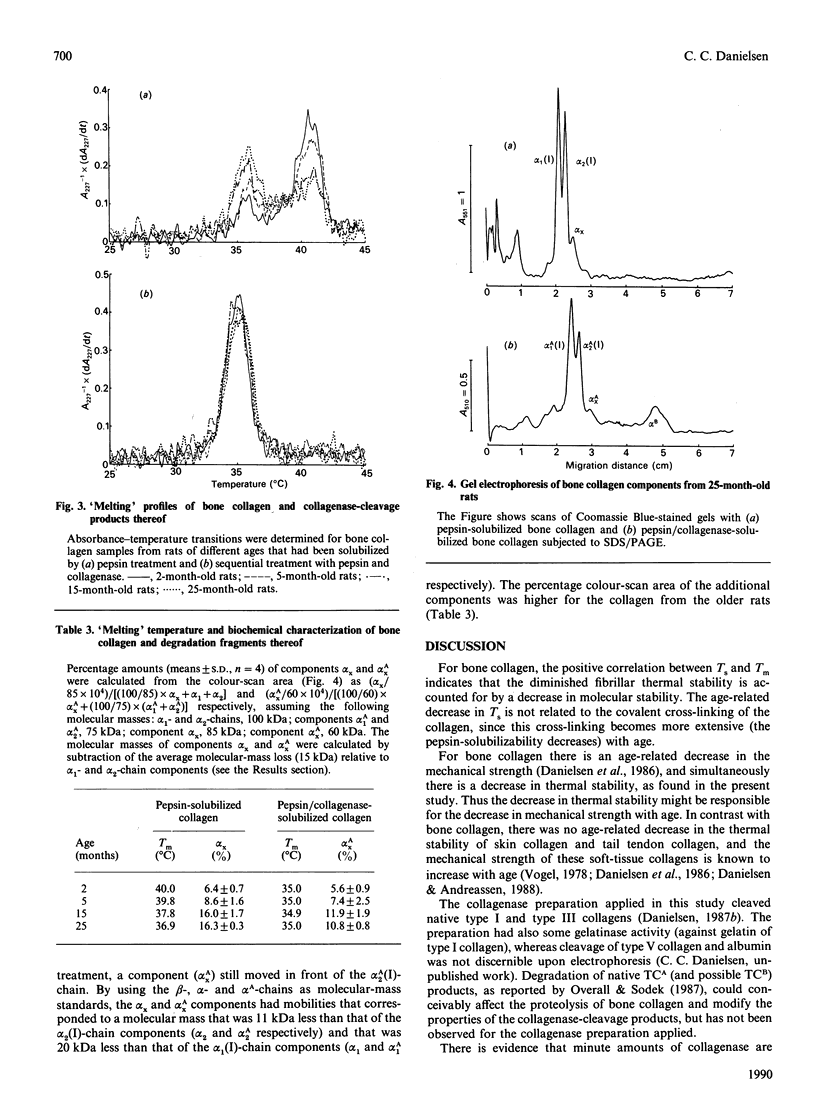
The shrinkage temperature (Ts) and the pepsin-solubilizability of collagen fibrils in bone matrix obtained from decalcified femur diaphysis from 2-, 5-, 15- and 25-month-old rats were found to decrease with age. Digestion with human fibroblast collagenase dissolved less than half of the collagen, whereas sequential treatment by pepsin followed by collagenase resulted in its complete dissolution. This result shows that collagenase and a telopeptide-cleaving enzyme, when acting in an appropriate sequence, have a great potential for the degradation of bone collagen. The 'melting' profile of the pepsin-solubilized collagen showed a biphasic transition with transition peak at 35.9 degrees C and 40.8 degrees C. With increasing age an increasing proportion of the collagen 'melted' in the transition peak at 35.9 degrees C (pre-transition), and the 'melting' temperature (Tm) of the collagen decreased in parallel with Ts in relation to age. Both Ts and Tm decreased by 3 degrees C in the age span investigated. The age-related change in Ts could therefore be accounted for by the decrease in molecular stability. The collagenase-cleavage products of the bone collagen obtained by the sequential treatment with pepsin and collagenase showed only one peak transition (at 35.1 degrees C), and the Tm for the products was independent of age. The results indicate that the pre-transition for the pepsin-solubilized collagen is due to an age-related decrease in thermal stability may have implications for the mechanical strength and turnover of the bone collagen. In contrast with bone collagen, soft-tissue collagen showed neither the age-dependency of thermal stability nor the characteristic biphasic 'melting' profile.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broek D. L., Madri J., Eikenberry E. F., Brodsky B. Characterization of the tissue form of type V collagen from chick bone. J Biol Chem. 1985 Jan 10;260(1):555–562. [PubMed] [Google Scholar]
- Danielsen C. C., Andreassen T. T. Mechanical properties of rat tail tendon in relation to proximal-distal sampling position and age. J Biomech. 1988;21(3):207–212. doi: 10.1016/0021-9290(88)90171-6. [DOI] [PubMed] [Google Scholar]
- Danielsen C. C., Andreassen T. T., Mosekilde L. Mechanical properties of collagen from decalcified rat femur in relation to age and in vitro maturation. Calcif Tissue Int. 1986 Aug;39(2):69–73. doi: 10.1007/BF02553293. [DOI] [PubMed] [Google Scholar]
- Danielsen C. C. Difference in thermal stability of type-I and type-II collagen from rat skin. Biochem J. 1982 Apr 1;203(1):323–326. doi: 10.1042/bj2030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen C. C. Mechanical properties of native and reconstituted rat tail tendon collagen upon maturation in vitro. Mech Ageing Dev. 1987 Sep 14;40(1):9–16. doi: 10.1016/0047-6374(87)90030-3. [DOI] [PubMed] [Google Scholar]
- Danielsen C. C. Precision method to determine denaturation temperature of collagen using ultraviolet difference spectroscopy. Coll Relat Res. 1982 Mar;2(2):143–150. doi: 10.1016/s0174-173x(82)80030-7. [DOI] [PubMed] [Google Scholar]
- Danielsen C. C. Reconstituted collagen fibrils. Fibrillar and molecular stability of the collagen upon maturation in vitro. Biochem J. 1984 Sep 15;222(3):663–668. doi: 10.1042/bj2220663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen C. C. Thermal stability of human-fibroblast-collagenase-cleavage products of type-I and type-III collagens. Biochem J. 1987 Nov 1;247(3):725–729. doi: 10.1042/bj2470725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen C. C. Thermal stability of reconstituted collagen fibrils. Shrinkage characteristics upon in vitro maturation. Mech Ageing Dev. 1981 Mar;15(3):269–278. doi: 10.1016/0047-6374(81)90135-4. [DOI] [PubMed] [Google Scholar]
- Dumas J., Hurion N., Weill R., Keil B. Collagenase in mineralized tissues of human teeth. FEBS Lett. 1985 Jul 22;187(1):51–55. doi: 10.1016/0014-5793(85)81212-6. [DOI] [PubMed] [Google Scholar]
- Eeckhout Y., Delaisse J. M. The role of collagenase in bone resorption. An overview. Pathol Biol (Paris) 1988 Nov;36(9):1139–1146. [PubMed] [Google Scholar]
- Eeckhout Y., Delaissé J. M., Vaes G. Direct extraction and assay of bone tissue collagenase and its relation to parathyroid-hormone-induced bone resorption. Biochem J. 1986 Nov 1;239(3):793–796. doi: 10.1042/bj2390793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Glimcher M. J. The dissolution of bovine and chicken bone collagens in concentrated formic acid. Calcif Tissue Res. 1974;15(2):125–132. doi: 10.1007/BF02059050. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Oguchi H. The hydroxypyridinium crosslinks of skeletal collagens: their measurement, properties and a proposed pathway of formation. Biochem Biophys Res Commun. 1980 Jan 29;92(2):403–410. doi: 10.1016/0006-291x(80)90347-2. [DOI] [PubMed] [Google Scholar]
- Fowler L. J., Bailey A. J. Current concepts of the crosslinking in bone collagen. Clin Orthop Relat Res. 1972;85:193–206. doi: 10.1097/00003086-197206000-00035. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971 Jun;41(2):510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- Kirsch E., Krieg T., Remberger K., Fendel H., Bruckner P., Müller P. K. Disorder of collagen metabolism in a patient with osteogenesis imperfecta (lethal type): increased degree of hydroxylation of lysine in collagen types I and III. Eur J Clin Invest. 1981 Feb;11(1):39–47. doi: 10.1111/j.1365-2362.1981.tb01763.x. [DOI] [PubMed] [Google Scholar]
- Mainardi C. L., Seyer J. M., Kang A. H. Type-specific collagenolysis: a type V collagen-degrading enzyme from macrophages. Biochem Biophys Res Commun. 1980 Dec 16;97(3):1108–1115. doi: 10.1016/0006-291x(80)91490-4. [DOI] [PubMed] [Google Scholar]
- Overall C. M., Sodek J. Initial characterization of a neutral metalloproteinase, active on native 3/4-collagen fragments, synthesized by ROS 17/2.8 osteoblastic cells, periodontal fibroblasts, and identified in gingival crevicular fluid. J Dent Res. 1987 Jul;66(7):1271–1282. doi: 10.1177/00220345870660071201. [DOI] [PubMed] [Google Scholar]
- Petrovic O. M., Miller E. J. An unusual pattern of peptide-bound lysine metabolism in collagen from an infant with lethal perinatal osteogenesis imperfecta. J Clin Invest. 1984 Jun;73(6):1569–1575. doi: 10.1172/JCI111363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes R. K., Miller E. J. Physicochemical characterization and molecular organization of the collagen A and B chains. Biochemistry. 1978 Aug 22;17(17):3442–3448. doi: 10.1021/bi00610a003. [DOI] [PubMed] [Google Scholar]
- Sakamoto S., Sakamoto M., Goldhaber P., Glimcher M. J. Isolation of tissue collagenase from homogenates of embryonic chick bones. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1102–1108. doi: 10.1016/0006-291x(73)90578-0. [DOI] [PubMed] [Google Scholar]
- Stoltz M., Furthmayr H., Timpl R. Increased lysine hydroxylation in rat bone and tendon collagen and localization of the additional residues. Biochim Biophys Acta. 1973 Jun 15;310(2):461–468. doi: 10.1016/0005-2795(73)90130-x. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Bauer E. A., Jeffrey J. J., Eisen A. Z. Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochemistry. 1977 Apr 19;16(8):1607–1615. doi: 10.1021/bi00627a013. [DOI] [PubMed] [Google Scholar]
- Vogel H. G. Influence of maturation and age on mechanical and biochemical parameters of connective tissue of various organs in the rat. Connect Tissue Res. 1978;6(3):161–166. doi: 10.3109/03008207809152626. [DOI] [PubMed] [Google Scholar]


