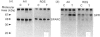Abstract
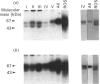
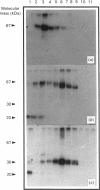
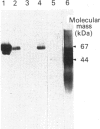
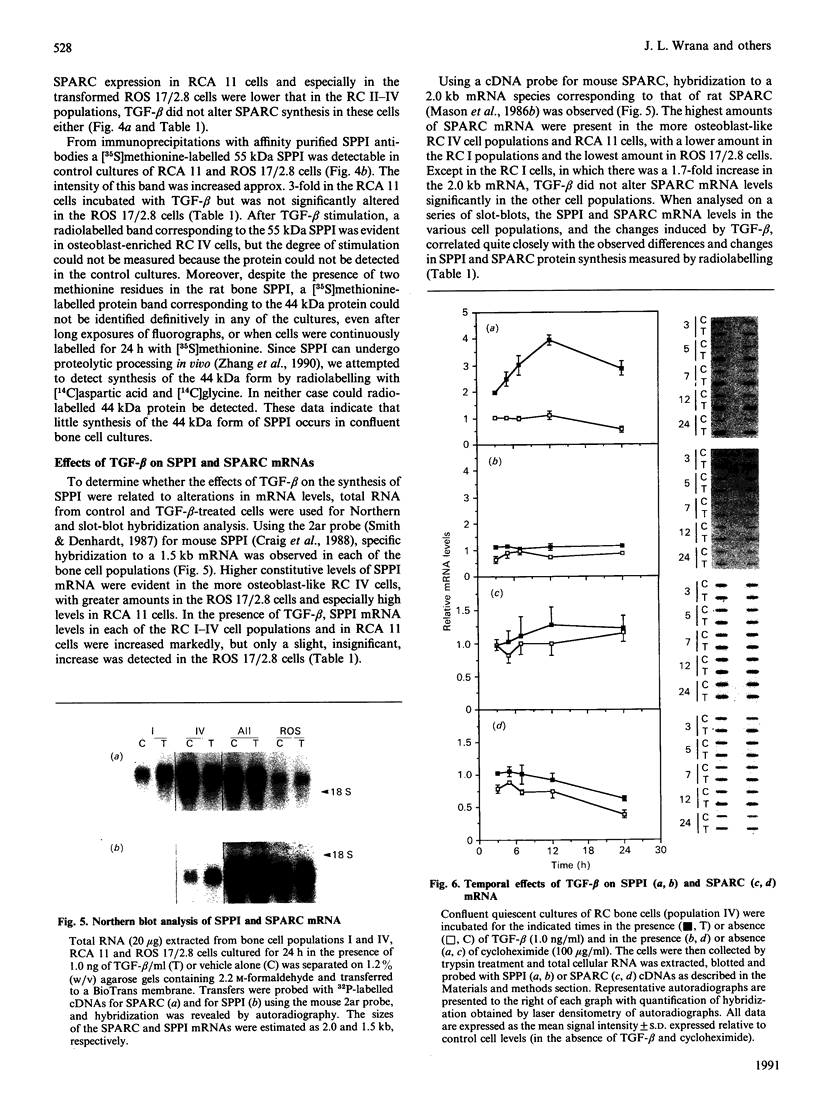
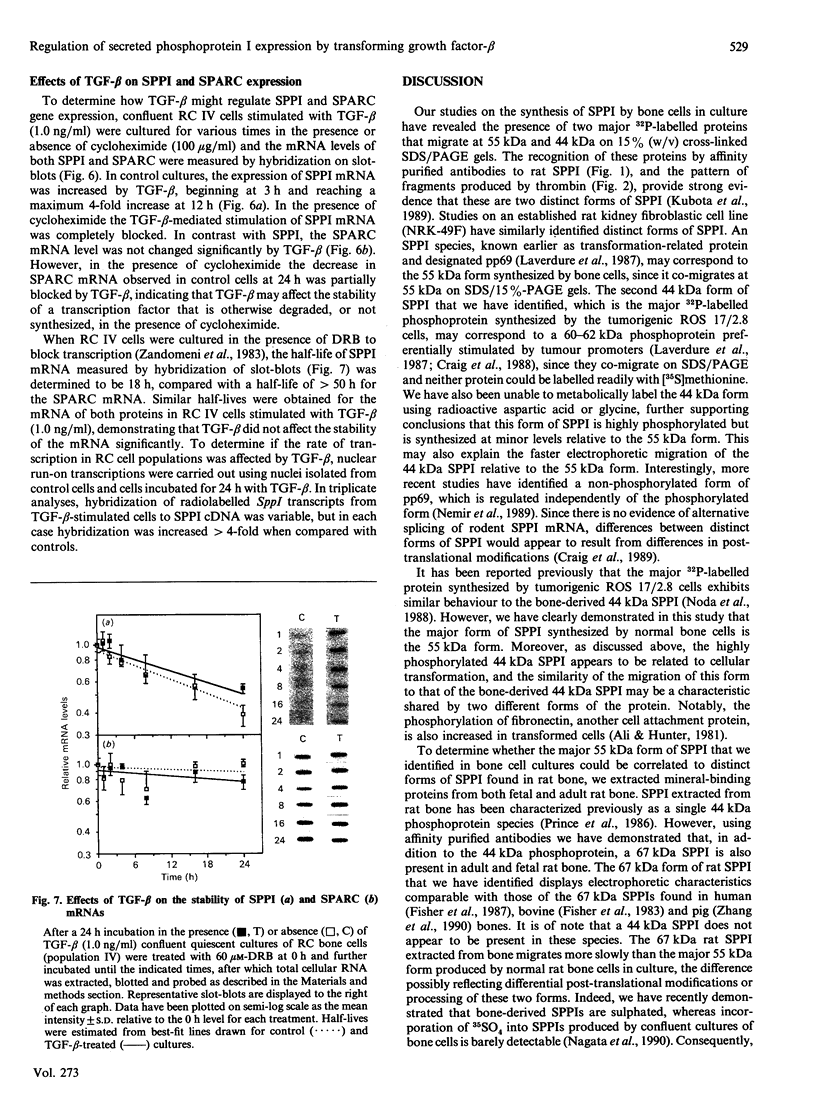
Secreted phosphoprotein I (SPPI; osteopontin), a highly phosphorylated form of which has been associated with cell transformation, is one of the major phosphorylated proteins in bone. Populations of rat bone cells derived from fetal calvariae, neonatal parietal bone and a rat osteosarcoma cell line (ROS 17/2.8) produce several forms of the protein, the major forms having apparent molecular masses of 55 and 44 kDa by SDS/PAGE on 15% (w/v) cross-linked gels and of 60 and 56 kDa on 10% gels. Northern blot analysis of SPPI mRNA using total cellular RNA revealed a single 1.5 kb mRNA species, indicating that the nascent protein chains of these phosphoproteins are identical. On treatment of the cells with transforming growth factor-beta (TGF-beta; 1 ng/ml), the levels of SPPI mRNA and the synthesis of the 55 kDa phosphoprotein, but not of the 44 kDa phosphoprotein, were increased by 1.8-4.5-fold in the normal osteoblastic cells, the stimulation first being evident at 3 h and reaching a maximum at 12 h. In the transformed ROS 17/2.8 cells, TGF-beta did not alter significantly the SPPI mRNA level or the synthesis of either the 55 kDa or the 44 kDa SPPI over the 24 h period studied. By comparison, neither the steady-state levels of SPARC (secreted protein, acidic, rich in cysteine) mRNA nor the synthesis of SPARC protein were affected significantly by the addition of TGF-beta to any of the osteoblastic bone cells. The half-lives for SPPI and SPARC mRNAs in the osteoblastic calvarial cells were calculated to be 18 h and greater than 50 h respectively, in both the presence and the absence of TGF-beta. Since the stability of the mRNA was unchanged by TGF-beta and the increased expression of SPPI mRNA could be blocked by cycloheximide, TGF-beta appears to increase transcription of the SppI gene indirectly by stimulating the synthesis of a protein that promotes transcription. These results demonstrate that several forms of SPPI are synthesized constitutively by bone cells, and that there are clear differences in the regulation of SppI gene expression by TGF-beta in normal bone cells compared with the tumorigenic ROS 17/2.8 cells. The differential responses of normal osteoblastic cells to TGF-beta in the expression of SPPI and the selective stimulation of specific forms of the SPPI protein may be important in bone repair and remodelling.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Hunter T. Structural comparison of fibronectins from normal and transformed cells. J Biol Chem. 1981 Jul 25;256(14):7671–7677. [PubMed] [Google Scholar]
- Antosz M. E., Bellows C. G., Aubin J. E. Effects of transforming growth factor beta and epidermal growth factor on cell proliferation and the formation of bone nodules in isolated fetal rat calvaria cells. J Cell Physiol. 1989 Aug;140(2):386–395. doi: 10.1002/jcp.1041400225. [DOI] [PubMed] [Google Scholar]
- Aubin J. E., Heersche J. N., Merrilees M. J., Sodek J. Isolation of bone cell clones with differences in growth, hormone responses, and extracellular matrix production. J Cell Biol. 1982 Feb;92(2):452–461. doi: 10.1083/jcb.92.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellows C. G., Aubin J. E., Heersche J. N., Antosz M. E. Mineralized bone nodules formed in vitro from enzymatically released rat calvaria cell populations. Calcif Tissue Int. 1986 Mar;38(3):143–154. doi: 10.1007/BF02556874. [DOI] [PubMed] [Google Scholar]
- Centrella M., Massagué J., Canalis E. Human platelet-derived transforming growth factor-beta stimulates parameters of bone growth in fetal rat calvariae. Endocrinology. 1986 Nov;119(5):2306–2312. doi: 10.1210/endo-119-5-2306. [DOI] [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987 Feb 25;262(6):2869–2874. [PubMed] [Google Scholar]
- Craig A. M., Nemir M., Mukherjee B. B., Chambers A. F., Denhardt D. T. Identification of the major phosphoprotein secreted by many rodent cell lines as 2ar/osteopontin: enhanced expression in H-ras-transformed 3T3 cells. Biochem Biophys Res Commun. 1988 Nov 30;157(1):166–173. doi: 10.1016/s0006-291x(88)80028-7. [DOI] [PubMed] [Google Scholar]
- Domenicucci C., Goldberg H. A., Hofmann T., Isenman D., Wasi S., Sodek J. Characterization of porcine osteonectin extracted from foetal calvariae. Biochem J. 1988 Jul 1;253(1):139–151. doi: 10.1042/bj2530139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen E. F., Colvard D. S., Berg N. J., Graham M. L., Mann K. G., Spelsberg T. C., Riggs B. L. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988 Jul 1;241(4861):84–86. doi: 10.1126/science.3388021. [DOI] [PubMed] [Google Scholar]
- Fet V., Dickinson M. E., Hogan B. L. Localization of the mouse gene for secreted phosphoprotein 1 (Spp-1) (2ar, osteopontin, bone sialoprotein 1, 44-kDa bone phosphoprotein, tumor-secreted phosphoprotein) to chromosome 5, closely linked to Ric (Rickettsia resistance). Genomics. 1989 Aug;5(2):375–377. doi: 10.1016/0888-7543(89)90074-8. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Hawkins G. R., Tuross N., Termine J. D. Purification and partial characterization of small proteoglycans I and II, bone sialoproteins I and II, and osteonectin from the mineral compartment of developing human bone. J Biol Chem. 1987 Jul 15;262(20):9702–9708. [PubMed] [Google Scholar]
- Fisher L. W., Whitson S. W., Avioli L. V., Termine J. D. Matrix sialoprotein of developing bone. J Biol Chem. 1983 Oct 25;258(20):12723–12727. [PubMed] [Google Scholar]
- Gorski J. P., Shimizu K. Isolation of new phosphorylated glycoprotein from mineralized phase of bone that exhibits limited homology to adhesive protein osteopontin. J Biol Chem. 1988 Nov 5;263(31):15938–15945. [PubMed] [Google Scholar]
- Komm B. S., Terpening C. M., Benz D. J., Graeme K. A., Gallegos A., Korc M., Greene G. L., O'Malley B. W., Haussler M. R. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science. 1988 Jul 1;241(4861):81–84. doi: 10.1126/science.3164526. [DOI] [PubMed] [Google Scholar]
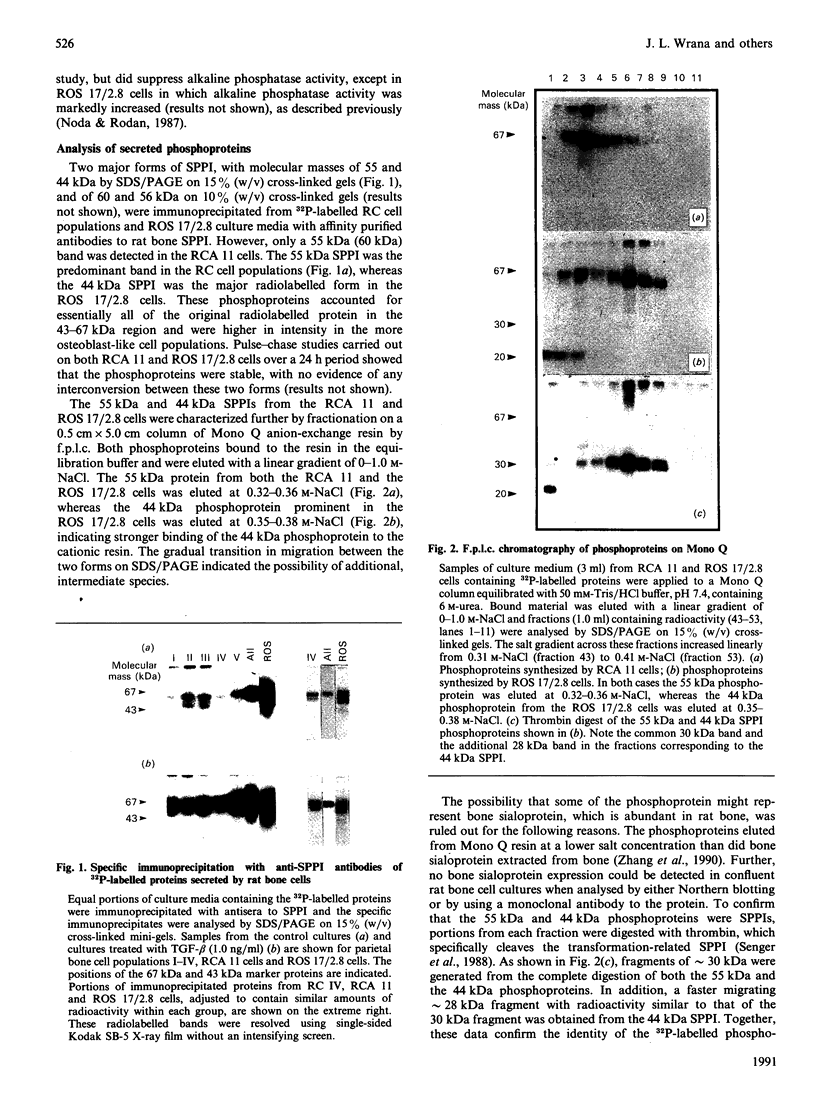
- Kubota T., Zhang Q., Wrana J. L., Ber R., Aubin J. E., Butler W. T., Sodek J. Multiple forms of SppI (secreted phosphoprotein, osteopontin) synthesized by normal and transformed rat bone cell populations: regulation by TGF-beta. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1453–1459. doi: 10.1016/0006-291x(89)90837-1. [DOI] [PubMed] [Google Scholar]
- Laverdure G. R., Banerjee D., Chackalaparampil I., Mukherjee B. B. Epidermal and transforming growth factors modulate secretion of a 69 kDa phosphoprotein in normal rat kidney fibroblasts. FEBS Lett. 1987 Oct 5;222(2):261–265. doi: 10.1016/0014-5793(87)80382-4. [DOI] [PubMed] [Google Scholar]
- Mark M. P., Butler W. T., Prince C. W., Finkelman R. D., Ruch J. V. Developmental expression of 44-kDa bone phosphoprotein (osteopontin) and bone gamma-carboxyglutamic acid (Gla)-containing protein (osteocalcin) in calcifying tissues of rat. Differentiation. 1988;37(2):123–136. doi: 10.1111/j.1432-0436.1988.tb00804.x. [DOI] [PubMed] [Google Scholar]
- Mark M. P., Prince C. W., Gay S., Austin R. L., Butler W. T. 44-kDal bone phosphoprotein (osteopontin) antigenicity at ectopic sites in newborn rats: kidney and nervous tissues. Cell Tissue Res. 1988 Jan;251(1):23–30. doi: 10.1007/BF00215443. [DOI] [PubMed] [Google Scholar]
- Mark M. P., Prince C. W., Oosawa T., Gay S., Bronckers A. L., Butler W. T. Immunohistochemical demonstration of a 44-KD phosphoprotein in developing rat bones. J Histochem Cytochem. 1987 Jul;35(7):707–715. doi: 10.1177/35.7.3295029. [DOI] [PubMed] [Google Scholar]
- Mason I. J., Murphy D., Münke M., Francke U., Elliott R. W., Hogan B. L. Developmental and transformation-sensitive expression of the Sparc gene on mouse chromosome 11. EMBO J. 1986 Aug;5(8):1831–1837. doi: 10.1002/j.1460-2075.1986.tb04434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason I. J., Taylor A., Williams J. G., Sage H., Hogan B. L. Evidence from molecular cloning that SPARC, a major product of mouse embryo parietal endoderm, is related to an endothelial cell 'culture shock' glycoprotein of Mr 43,000. EMBO J. 1986 Jul;5(7):1465–1472. doi: 10.1002/j.1460-2075.1986.tb04383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T., Todescan R., Goldberg H. A., Zhang Q., Sodek J. Sulphation of secreted phosphoprotein I (SPPI, osteopontin) is associated with mineralized tissue formation. Biochem Biophys Res Commun. 1989 Nov 30;165(1):234–240. doi: 10.1016/0006-291x(89)91059-0. [DOI] [PubMed] [Google Scholar]
- Nemir M., DeVouge M. W., Mukherjee B. B. Normal rat kidney cells secrete both phosphorylated and nonphosphorylated forms of osteopontin showing different physiological properties. J Biol Chem. 1989 Oct 25;264(30):18202–18208. [PubMed] [Google Scholar]
- Noda M., Rodan G. A. Type beta transforming growth factor (TGF beta) regulation of alkaline phosphatase expression and other phenotype-related mRNAs in osteoblastic rat osteosarcoma cells. J Cell Physiol. 1987 Dec;133(3):426–437. doi: 10.1002/jcp.1041330303. [DOI] [PubMed] [Google Scholar]
- Noda M., Yoon K., Prince C. W., Butler W. T., Rodan G. A. Transcriptional regulation of osteopontin production in rat osteosarcoma cells by type beta transforming growth factor. J Biol Chem. 1988 Sep 25;263(27):13916–13921. [PubMed] [Google Scholar]
- Nomura S., Wills A. J., Edwards D. R., Heath J. K., Hogan B. L. Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol. 1988 Feb;106(2):441–450. doi: 10.1083/jcb.106.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. The primary structure of a cell-binding bone sialoprotein. J Biol Chem. 1988 Dec 25;263(36):19430–19432. [PubMed] [Google Scholar]
- Otsuka K., Yao K. L., Wasi S., Tung P. S., Aubin J. E., Sodek J., Termine J. D. Biosynthesis of osteonectin by fetal porcine calvarial cells in vitro. J Biol Chem. 1984 Aug 10;259(15):9805–9812. [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem. 1989 Jan 25;264(3):1860–1869. [PubMed] [Google Scholar]
- Pfeilschifter J., Mundy G. R. Modulation of type beta transforming growth factor activity in bone cultures by osteotropic hormones. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2024–2028. doi: 10.1073/pnas.84.7.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince C. W., Butler W. T. 1,25-Dihydroxyvitamin D3 regulates the biosynthesis of osteopontin, a bone-derived cell attachment protein, in clonal osteoblast-like osteosarcoma cells. Coll Relat Res. 1987 Sep;7(4):305–313. doi: 10.1016/s0174-173x(87)80036-5. [DOI] [PubMed] [Google Scholar]
- Prince C. W., Oosawa T., Butler W. T., Tomana M., Bhown A. S., Bhown M., Schrohenloher R. E. Isolation, characterization, and biosynthesis of a phosphorylated glycoprotein from rat bone. J Biol Chem. 1987 Feb 25;262(6):2900–2907. [PubMed] [Google Scholar]
- Rao L. G., Ng B., Brunette D. M., Heersche J. N. Parathyroid hormone- and prostaglandin E1-response in a selected population of bone cells after repeated subculture and storage at -80C. Endocrinology. 1977 May;100(5):1233–1241. doi: 10.1210/endo-100-5-1233. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Gracey C. F., Papadopoulos A., Tenen D. G. Secreted phosphoproteins associated with neoplastic transformation: close homology with plasma proteins cleaved during blood coagulation. Cancer Res. 1988 Oct 15;48(20):5770–5774. [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Papadopoulos A., Tenen D. G. Purification of a human milk protein closely similar to tumor-secreted phosphoproteins and osteopontin. Biochim Biophys Acta. 1989 Jun 13;996(1-2):43–48. doi: 10.1016/0167-4838(89)90092-7. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A. Secreted phosphoprotein markers for neoplastic transformation of human epithelial and fibroblastic cells. Cancer Res. 1985 Nov;45(11 Pt 2):5818–5823. [PubMed] [Google Scholar]
- Seyedin S. M., Segarini P. R., Rosen D. M., Thompson A. Y., Bentz H., Graycar J. Cartilage-inducing factor-B is a unique protein structurally and functionally related to transforming growth factor-beta. J Biol Chem. 1987 Feb 15;262(5):1946–1949. [PubMed] [Google Scholar]
- Smith J. H., Denhardt D. T. Molecular cloning of a tumor promoter-inducible mRNA found in JB6 mouse epidermal cells: induction is stable at high, but not at low, cell densities. J Cell Biochem. 1987 May;34(1):13–22. doi: 10.1002/jcb.240340103. [DOI] [PubMed] [Google Scholar]
- Somerman M. J., Prince C. W., Butler W. T., Foster R. A., Moehring J. M., Sauk J. J. Cell attachment activity of the 44 kilodalton bone phosphoprotein is not restricted to bone cells. Matrix. 1989 Jan;9(1):49–54. doi: 10.1016/s0934-8832(89)80018-6. [DOI] [PubMed] [Google Scholar]
- Somerman M. J., Prince C. W., Sauk J. J., Foster R. A., Butler W. T. Mechanism of fibroblast attachment to bone extracellular matrix: role of a 44 kilodalton bone phosphoprotein. J Bone Miner Res. 1987 Jun;2(3):259–265. doi: 10.1002/jbmr.5650020313. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termine J. D., Belcourt A. B., Conn K. M., Kleinman H. K. Mineral and collagen-binding proteins of fetal calf bone. J Biol Chem. 1981 Oct 25;256(20):10403–10408. [PubMed] [Google Scholar]
- Tung P. S., Domenicucci C., Wasi S., Sodek J. Specific immunohistochemical localization of osteonectin and collagen types I and III in fetal and adult porcine dental tissues. J Histochem Cytochem. 1985 Jun;33(6):531–540. doi: 10.1177/33.6.3889139. [DOI] [PubMed] [Google Scholar]
- Wasi S., Otsuka K., Yao K. L., Tung P. S., Aubin J. E., Sodek J., Termine J. D. An osteonectinlike protein in porcine periodontal ligament and its synthesis by periodontal ligament fibroblasts. Can J Biochem Cell Biol. 1984 Jun;62(6):470–478. doi: 10.1139/o84-064. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Maeno M., Hawrylyshyn B., Yao K. L., Domenicucci C., Sodek J. Differential effects of transforming growth factor-beta on the synthesis of extracellular matrix proteins by normal fetal rat calvarial bone cell populations. J Cell Biol. 1988 Mar;106(3):915–924. doi: 10.1083/jcb.106.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana J. L., Sodek J., Ber R. L., Bellows C. G. The effects of platelet-derived transforming growth factor beta on normal human diploid gingival fibroblasts. Eur J Biochem. 1986 Aug 15;159(1):69–76. doi: 10.1111/j.1432-1033.1986.tb09834.x. [DOI] [PubMed] [Google Scholar]
- Yoon K., Buenaga R., Rodan G. A. Tissue specificity and developmental expression of rat osteopontin. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1129–1136. doi: 10.1016/s0006-291x(87)80250-4. [DOI] [PubMed] [Google Scholar]
- Zandomeni R., Bunick D., Ackerman S., Mittleman B., Weinmann R. Mechanism of action of DRB. III. Effect on specific in vitro initiation of transcription. J Mol Biol. 1983 Jul 5;167(3):561–574. doi: 10.1016/s0022-2836(83)80098-9. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Domenicucci C., Goldberg H. A., Wrana J. L., Sodek J. Characterization of fetal porcine bone sialoproteins, secreted phosphoprotein I (SPPI, osteopontin), bone sialoprotein, and a 23-kDa glycoprotein. Demonstration that the 23-kDa glycoprotein is derived from the carboxyl terminus of SPPI. J Biol Chem. 1990 May 5;265(13):7583–7589. [PubMed] [Google Scholar]