Abstract
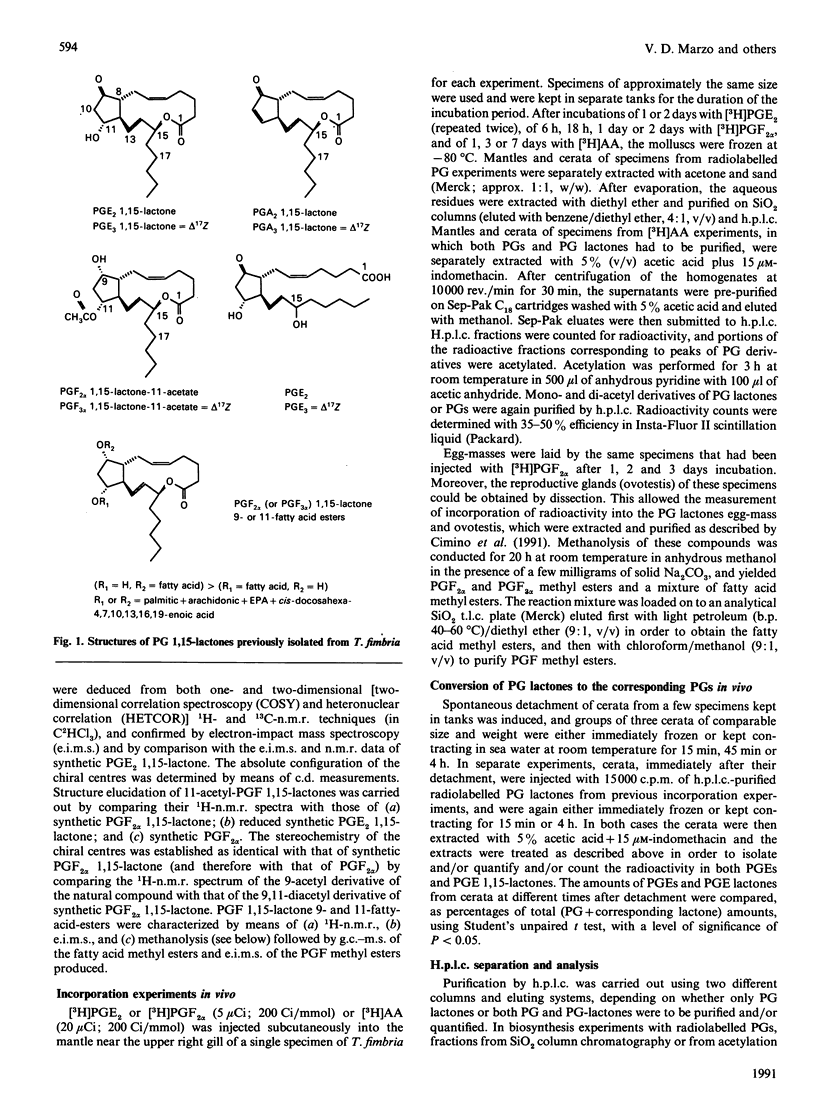
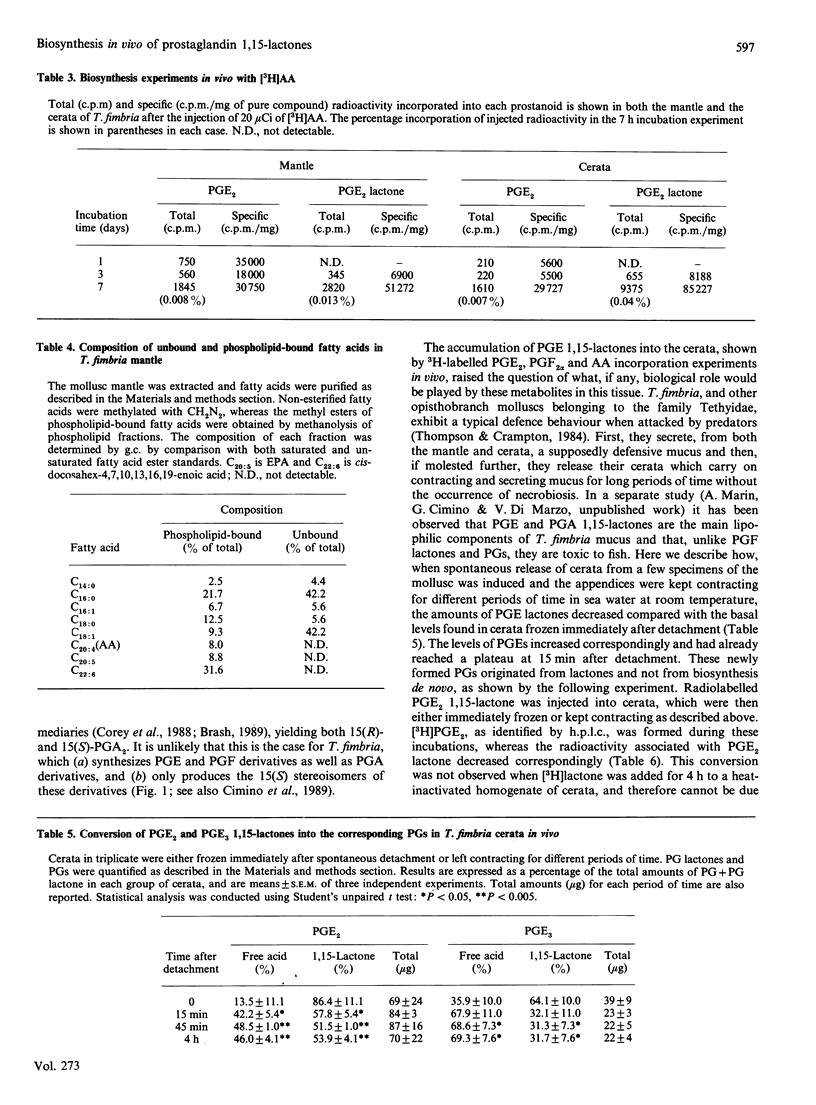
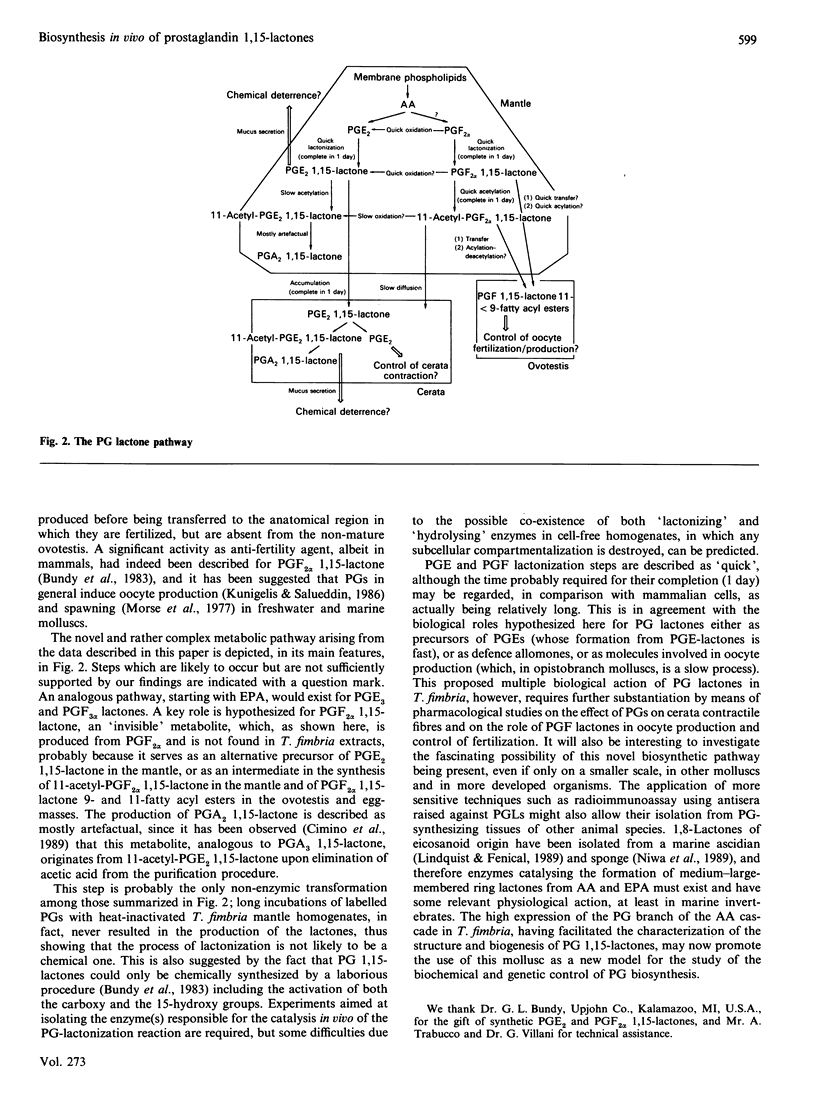
The discovery of high levels of prostaglandin (PG) 1,15-lactones of both the E and F series and their co-existence with PGs has been recently described in the opisthobranch mollusc Tethys fimbria. The present study was undertaken in order to investigate the biosynthesis of these novel natural PG derivatives in vivo using radiolabelled precursors, and to gain a preliminary understanding of their biological role. PGE2 1,15-lactone was shown to be produced from both PGE2 and PGF2 alpha in the mollusc mantle and appeared to be quickly transferred to the mollusc dorsal appendices (cerata). The detachment of the latter during the typical defence behaviour of T. fimbria was accompanied by the conversion of PGE2 and PGE3 1,15-lactones back to the corresponding PGs. Both PGE2 and PGE2 1,15-lactone were also shown to be biosynthesized from arachidonic acid. Lactones of the F series were present as 11-acetyl derivatives in T. fimbria mantle and as 9- and 11-fatty acyl esters in the mollusc egg-mass and reproductive gland, and their biosynthesis from PGF2 alpha was demonstrated in all of these tissues. A multiple biological role of PG 1,15-lactones in T. fimbria defensive behaviour, smooth muscle contraction and egg production/fertilization control is hypothesized. The high amounts of PG derivatives found in T. fimbria and the biosynthetic studies described herein indicate that this marine mollusc may be a useful model for future studies on PG biosynthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bundy G. L., Peterson D. C., Cornette J. C., Miller W. L., Spilman C. H., Wilks J. W. Synthesis and biological activity of prostaglandin lactones. J Med Chem. 1983 Aug;26(8):1089–1099. doi: 10.1021/jm00362a001. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Duncan H., Hooker N., Morse A. Hydrogen peroxide induces spawning in mollusks, with activation of prostaglandin endoperoxide synthetase. Science. 1977 Apr 15;196(4287):298–300. doi: 10.1126/science.403609. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Kayne M., Tidyman M., Anderson S. Capacity for biosynthesis of prostaglandin-related compounds: distribution and properties of the rate-limiting enzyme in hydrocorals, gorgoniants, and other coelenterates of the Caribbean and Pacific. Biol Bull. 1978 Jun;154(3):440–452. doi: 10.2307/1541070. [DOI] [PubMed] [Google Scholar]
- Saintsing D. G., Hwang D. H., Dietz T. H. Production of prostaglandins E2 and F2 alpha in the freshwater mussel Ligumia subrostrata: relation to sodium transport. J Pharmacol Exp Ther. 1983 Aug;226(2):455–461. [PubMed] [Google Scholar]
- Samuelsson B., Granström E., Green K., Hamberg M., Hammarström S. Prostaglandins. Annu Rev Biochem. 1975;44:669–695. doi: 10.1146/annurev.bi.44.070175.003321. [DOI] [PubMed] [Google Scholar]
- Weinheimer A. J., Spraggins R. L. The occurrence of two new prostaglandin derivatives (15-epi-PGA2 and its acetate, methyl ester) in the gorgonian Plexaura homomalla chemistry of coelenterates. XV. Tetrahedron Lett. 1969 Dec;(59):5185–5188. doi: 10.1016/s0040-4039(01)88918-8. [DOI] [PubMed] [Google Scholar]


