Abstract
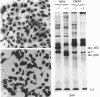
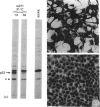
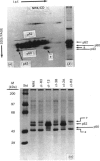
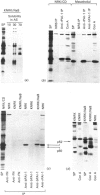
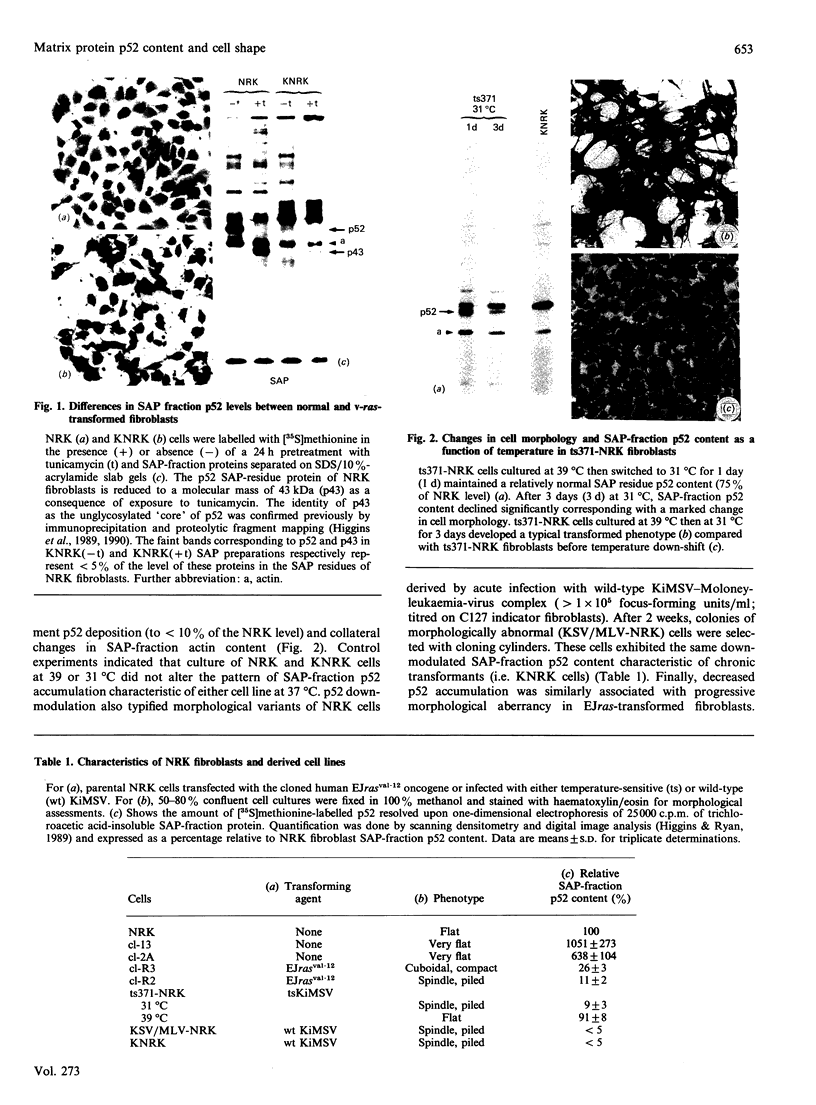
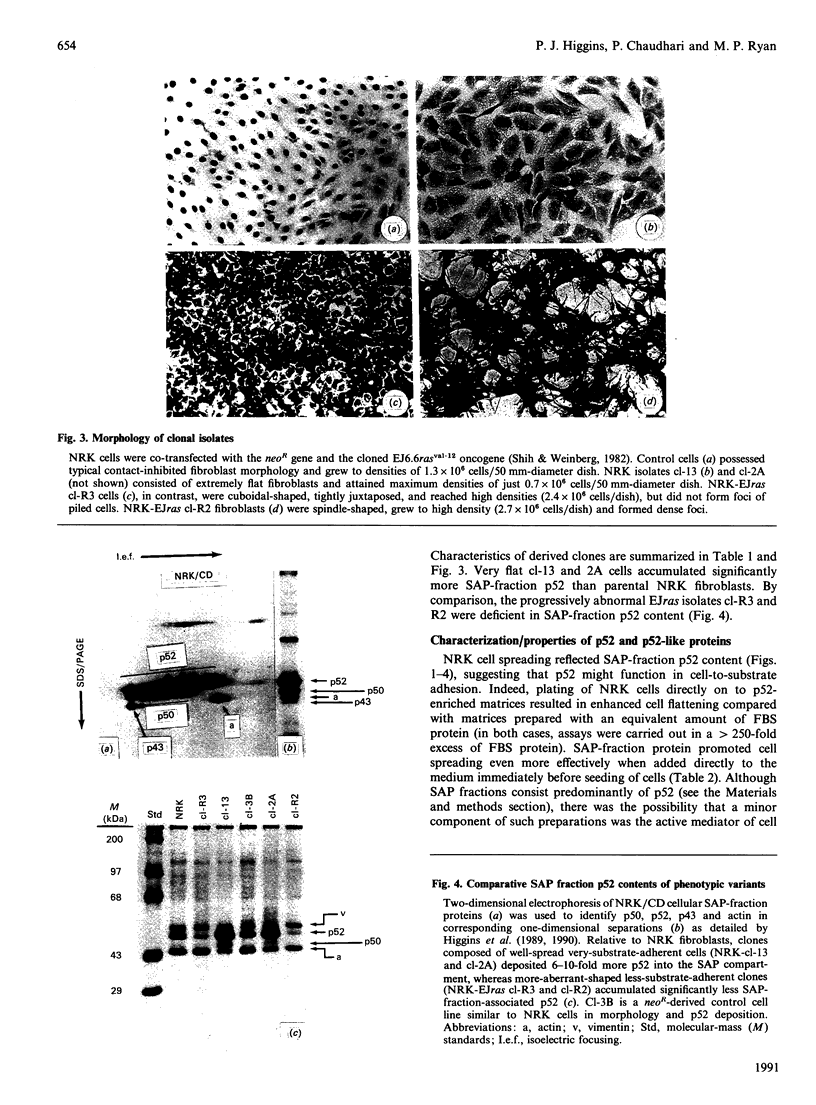
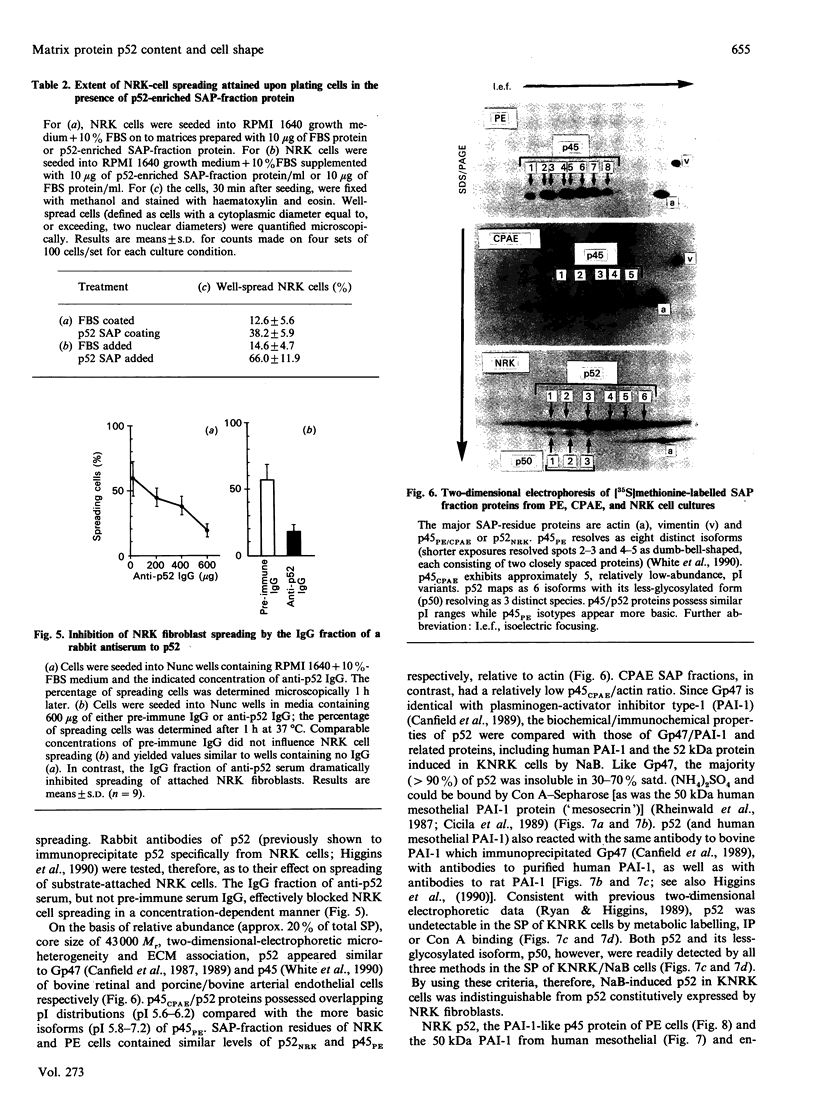
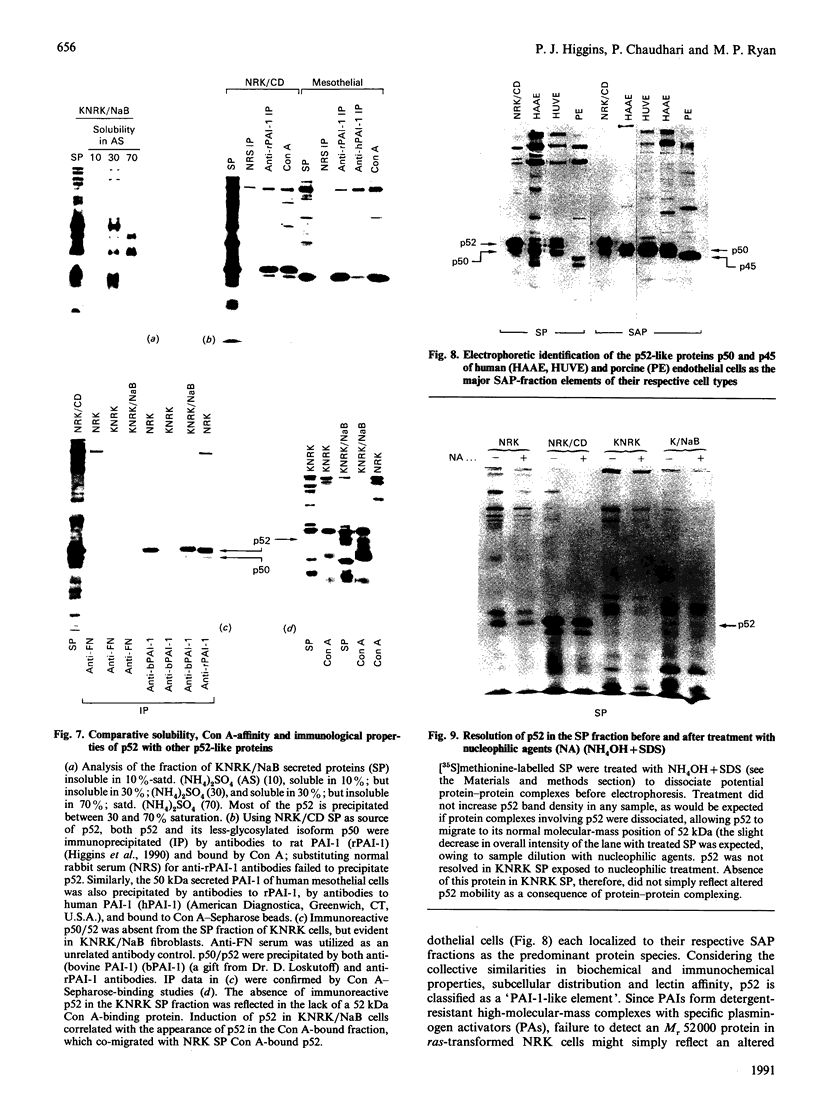
The 52 kDa transformation-sensitive protein p52 was previously identified as a major substrate-associated component of normal rat kidney (NRK) fibroblasts [Higgins & Ryan (1989) Biochem. J. 257, 173-182]. p52 selectively localized to cellular fractions enriched in substrate focal-contact sites and associated ventral undersurface elements. Rapid attachment/spreading of NRK cells on to prepared p52 matrices and inhibition of fibroblast spreading by antibodies to p52 indicated that this protein participates in shape determination or cell-to-substrate adhesion. NRK cells transformed with Kirsten murine sarcoma virus (KiMSV), with a temperature-sensitive mutant (ts-371 KiMSV) and maintained at the permissive temperature, or with the cloned EJrasval.12 oncogene, exhibited down-regulated accumulation of p52 in the ventral undersurface region. Immunochemical, lectin-affinity and electrophoretic analyses indicated that p52 shares considerable sequence similarity with plasminogen-activator inhibitor type-1, which is consistent with its subcellular localization and likely morphoregulatory activity. The marked down-regulation of p52 expression seen in four different ras-mediated transformation systems, its induction prior to butyrate-induced morphological reorganization in KiMSV-transformed cells, and the morphological consequences of exogenously added p52 or p52 antibodies on NRK fibroblasts suggest that this protein probably functions in cell-shape regulation. Abrogation of p52 matrix accumulation typically seen in ras transformants may contribute, therefore, to the aberrant cytoarchitecture characteristic of malignant fibroblasts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Mautner V., Lanza R., Hynes R. O. Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell. 1977 May;11(1):115–126. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- Altenburg B. C., Via D. P., Steiner S. H. Modification of the phenotype of murine sarcoma virus-transformed cells by sodium butyrate. Effects on morphology and cytoskeletal elements. Exp Cell Res. 1976 Oct 15;102(2):223–231. doi: 10.1016/0014-4827(76)90036-7. [DOI] [PubMed] [Google Scholar]
- Aplin J. D., Hughes R. C., Jaffe C. L., Sharon N. Reversible cross-linking of cellular components of adherent fibroblasts to fibronectin and lectin-coated substrata. Exp Cell Res. 1981 Aug;134(2):488–494. doi: 10.1016/0014-4827(81)90453-5. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Scheinbuks J., Lin P. H., Traylor M., Bruce R. Isolation and interrelationships of the multiple molecular tissue-type and urokinase-type plasminogen activator forms produced by cultured human umbilical vein endothelial cells. J Biol Chem. 1988 Oct 15;263(29):15129–15138. [PubMed] [Google Scholar]
- Bruzdzinski C. J., Riordan-Johnson M., Nordby E. C., Suter S. M., Gelehrter T. D. Isolation and characterization of the rat plasminogen activator inhibitor-1 gene. J Biol Chem. 1990 Feb 5;265(4):2078–2085. [PubMed] [Google Scholar]
- Canfield A. E., Schor A. M., Loskutoff D. J., Schor S. L., Grant M. E. Plasminogen activator inhibitor-type I is a major biosynthetic product of retinal microvascular endothelial cells and pericytes in culture. Biochem J. 1989 Apr 15;259(2):529–535. doi: 10.1042/bj2590529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield A. E., Schor A. M., West D. C., Schor S. L., Grant M. E. Identification and partial characterization of two major proteins of Mr 47,000 synthesized by bovine retinal endothelial cells in culture. Biochem J. 1987 Aug 15;246(1):121–129. doi: 10.1042/bj2460121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. T., Wang J., Hasegawa T., Yamada S. S., Yamada K. M. Regulation of fibronectin receptor distribution by transformation, exogenous fibronectin, and synthetic peptides. J Cell Biol. 1986 Nov;103(5):1649–1661. doi: 10.1083/jcb.103.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicila G. T., O'Connell T. M., Hahn W. C., Rheinwald J. G. Cloned cDNA sequence for the human mesothelial protein 'mesosecrin' discloses its identity as a plasminogen activator inhibitor (PAI-1) and a recent evolutionary change in transcript processing. J Cell Sci. 1989 Sep;94(Pt 1):1–10. doi: 10.1242/jcs.94.1.1. [DOI] [PubMed] [Google Scholar]
- Cohen R. L., Niclas J., Lee W. M., Wun T. C., Crowley C. W., Levinson A. D., Sadler J. E., Shuman M. A. Effects of cellular transformation on expression of plasminogen activator inhibitors 1 and 2. Evidence for independent regulation. J Biol Chem. 1989 May 15;264(14):8375–8383. [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Damsky C. H., Knudsen K. A., Bradley D., Buck C. A., Horwitz A. F. Distribution of the cell substratum attachment (CSAT) antigen on myogenic and fibroblastic cells in culture. J Cell Biol. 1985 May;100(5):1528–1539. doi: 10.1083/jcb.100.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Declerck P. J., De Mol M., Alessi M. C., Baudner S., Pâques E. P., Preissner K. T., Müller-Berghaus G., Collen D. Purification and characterization of a plasminogen activator inhibitor 1 binding protein from human plasma. Identification as a multimeric form of S protein (vitronectin). J Biol Chem. 1988 Oct 25;263(30):15454–15461. [PubMed] [Google Scholar]
- Edelman G. M. Morphoregulatory molecules. Biochemistry. 1988 May 17;27(10):3533–3543. doi: 10.1021/bi00410a001. [DOI] [PubMed] [Google Scholar]
- Hayman E. G., Engvall E., Ruoslahti E. Concomitant loss of cell surface fibronectin and laminin from transformed rat kidney cells. J Cell Biol. 1981 Feb;88(2):352–357. doi: 10.1083/jcb.88.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins P. J., Ryan M. P. Biochemical localization of the transformation-sensitive 52 kDa (p52) protein to the substratum contact regions of cultured rat fibroblasts. Butyrate induction, characterization, and quantification of p52 in v-ras transformed cells. Biochem J. 1989 Jan 1;257(1):173–182. doi: 10.1042/bj2570173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins P. J., Ryan M. P., Chaudhari P. Cytochalasin D-mediated hyperinduction of the substrate-associated 52-kilodalton protein p52 in rat kidney fibroblasts. J Cell Physiol. 1989 May;139(2):407–417. doi: 10.1002/jcp.1041390225. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Ryan M. P., Zeheb R., Gelehrter T. D., Chaudhari P. p52 induction by cytochalasin D in rat kidney fibroblasts: homologies between p52 and plasminogen activator inhibitor type-1. J Cell Physiol. 1990 May;143(2):321–329. doi: 10.1002/jcp.1041430216. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Imler J. L., Schatz C., Wasylyk C., Chatton B., Wasylyk B. A Harvey-ras responsive transcription element is also responsive to a tumour-promoter and to serum. Nature. 1988 Mar 17;332(6161):275–278. doi: 10.1038/332275a0. [DOI] [PubMed] [Google Scholar]
- Jaggi R., Friis R., Groner B. Oncogenes modulate cellular gene expression and repress glucocorticoid regulated gene transcription. J Steroid Biochem. 1988 May;29(5):457–463. doi: 10.1016/0022-4731(88)90179-3. [DOI] [PubMed] [Google Scholar]
- Laiho M., Keski-Oja J. Growth factors in the regulation of pericellular proteolysis: a review. Cancer Res. 1989 May 15;49(10):2533–2553. [PubMed] [Google Scholar]
- Laiho M., Saksela O., Keski-Oja J. Transforming growth factor-beta induction of type-1 plasminogen activator inhibitor. Pericellular deposition and sensitivity to exogenous urokinase. J Biol Chem. 1987 Dec 25;262(36):17467–17474. [PubMed] [Google Scholar]
- Levin E. G., Santell L. Association of a plasminogen activator inhibitor (PAI-1) with the growth substratum and membrane of human endothelial cells. J Cell Biol. 1987 Dec;105(6 Pt 1):2543–2549. doi: 10.1083/jcb.105.6.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M., Lund L. R., Riccio A., Skouv J., Nielsen L. S., Stacey S. N., Danø K., Andreasen P. A. Plasminogen activator inhibitor type-1 protein, mRNA and gene transcription are increased by phorbol esters in human rhabdomyosarcoma cells. J Biol Chem. 1988 Oct 25;263(30):15688–15693. [PubMed] [Google Scholar]
- Neyfakh A. A., Jr, Svitkina T. M. Isolation of focal contact membrane using saponin. Exp Cell Res. 1983 Dec;149(2):582–586. doi: 10.1016/0014-4827(83)90369-5. [DOI] [PubMed] [Google Scholar]
- Norris W. E. Evidence for a second class of membrane glycoprotein involved in cell adhesion. J Cell Sci. 1989 Aug;93(Pt 4):631–640. doi: 10.1242/jcs.93.4.631. [DOI] [PubMed] [Google Scholar]
- Oesch B., Birchmeier W. New surface component of fibroblast's focal contacts identified by a monoclonal antibody. Cell. 1982 Dec;31(3 Pt 2):671–679. doi: 10.1016/0092-8674(82)90322-1. [DOI] [PubMed] [Google Scholar]
- Phillips P. G., Higgins P. J., Malik A. B., Tsan M. F. Effect of hyperoxia on the cytoarchitecture of cultured endothelial cells. Am J Pathol. 1988 Jul;132(1):59–72. [PMC free article] [PubMed] [Google Scholar]
- Plantefaber L. C., Hynes R. O. Changes in integrin receptors on oncogenically transformed cells. Cell. 1989 Jan 27;56(2):281–290. doi: 10.1016/0092-8674(89)90902-1. [DOI] [PubMed] [Google Scholar]
- Pöllänen J., Hedman K., Nielsen L. S., Danø K., Vaheri A. Ultrastructural localization of plasma membrane-associated urokinase-type plasminogen activator at focal contacts. J Cell Biol. 1988 Jan;106(1):87–95. doi: 10.1083/jcb.106.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöllänen J., Saksela O., Salonen E. M., Andreasen P., Nielsen L., Danø K., Vaheri A. Distinct localizations of urokinase-type plasminogen activator and its type 1 inhibitor under cultured human fibroblasts and sarcoma cells. J Cell Biol. 1987 Apr;104(4):1085–1096. doi: 10.1083/jcb.104.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald J. G., Jorgensen J. L., Hahn W. C., Terpstra A. J., O'Connell T. M., Plummer K. K. Mesosecrin: a secreted glycoprotein produced in abundance by human mesothelial, endothelial, and kidney epithelial cells in culture. J Cell Biol. 1987 Feb;104(2):263–275. doi: 10.1083/jcb.104.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. P., Borenfreund E., Higgins P. J. Cytoarchitectural analysis of epithelial sheets formed in vitro by hepatic tumor cells possessing defined intermediate-sized filament cytoskeletal abnormalities. Am J Pathol. 1989 Feb;134(2):447–456. [PMC free article] [PubMed] [Google Scholar]
- Ryan M. P., Higgins P. J. Cytoarchitecture of Kirsten sarcoma virus-transformed rat kidney fibroblasts: butyrate-induced reorganization within the actin microfilament network. J Cell Physiol. 1988 Oct;137(1):25–34. doi: 10.1002/jcp.1041370104. [DOI] [PubMed] [Google Scholar]
- Ryan M. P., Higgins P. J. Sodium-n-butyrate induces secretion and substrate accumulation of p52 in Kirsten sarcoma virus-transformed rat kidney fibroblasts. Int J Biochem. 1989;21(1):31–37. doi: 10.1016/0020-711x(89)90024-4. [DOI] [PubMed] [Google Scholar]
- Santarén J. F., Bravo R. Immediate induction of a 45 K secreted glycoprotein by serum and growth factors in quiescent mouse 3T3 cells. Two-dimensional gel analysis. Exp Cell Res. 1987 Feb;168(2):494–506. doi: 10.1016/0014-4827(87)90022-x. [DOI] [PubMed] [Google Scholar]
- Satake M., Ibaraki T., Yamaguchi Y., Ito Y. Loss of responsiveness of an AP1-related factor, PEBP1, to 12-O-tetradecanoylphorbol-13-acetate after transformation of NIH 3T3 cells by the Ha-ras oncogene. J Virol. 1989 Sep;63(9):3669–3677. doi: 10.1128/jvi.63.9.3669-3677.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Weinberg R. A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982 May;29(1):161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scolnick E. M. p21 of Kirsten murine sarcoma virus is thermolabile in a viral mutant temperature sensitive for the maintenance of transformation. J Virol. 1979 Aug;31(2):546–546. doi: 10.1128/jvi.31.2.546-546.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistonen L., Keski-Oja J., Ulmanen I., Hölttä E., Wikgren B. J., Alitalo K. Dose effects of transfected c-Ha-rasVal 12 oncogene in transformed cell clones. Exp Cell Res. 1987 Feb;168(2):518–530. doi: 10.1016/0014-4827(87)90024-3. [DOI] [PubMed] [Google Scholar]
- Thalacker F. W., Nilsen-Hamilton M. Specific induction of secreted proteins by transforming growth factor-beta and 12-O-tetradecanoylphorbol-13-acetate. Relationship with an inhibitor of plasminogen activator. J Biol Chem. 1987 Feb 15;262(5):2283–2290. [PubMed] [Google Scholar]
- Wagner D. D., Ivatt R., Destree A. T., Hynes R. O. Similarities and differences between the fibronectins of normal and transformed hamster cells. J Biol Chem. 1981 Nov 25;256(22):11708–11715. [PubMed] [Google Scholar]
- White J. E., Tsan M. F., Phillips P. G., Higgins P. J. The substrate-associated protein p45 of porcine endothelial cells: multiple isoforms, cytoskeletal-like properties and induction by hyperoxic stress. Int J Biochem. 1990;22(10):1159–1164. doi: 10.1016/0020-711x(90)90115-j. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]