Abstract
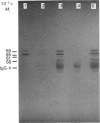
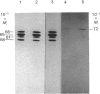
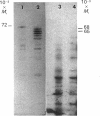
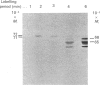
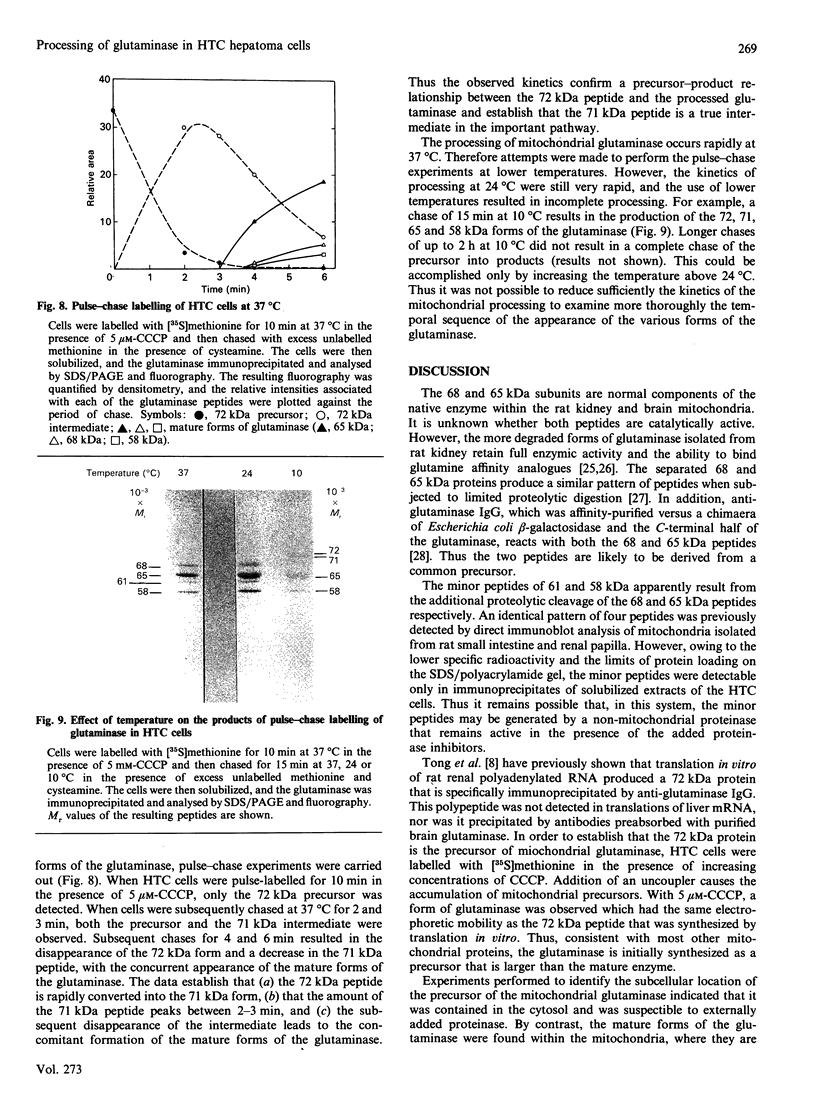
Rat HTC hepatoma cells were used to characterize the biosynthesis and processing of the renal isoenzyme of the mitochondrial glutaminase. Immunoblot analysis indicated that mitochondria isolated from HTC cells contained two prominent glutaminase peptides of 68 and 65 kDa and two minor peptides of 61 and 58 kDa. When the cells were labelled with [35S]methionine, the glutaminase-specific antibodies precipitated the same four polypeptides. However, when labelled in the presence of 5 microM-carbonyl cyanide m-chlorophenylhydrazone, an uncoupler of oxidative phosphorylation, only a 72 kDa cytoplasmic precursor of the mitochondrial glutaminase was immunoprecipitated. A comparison of the peptides generated by partial proteolysis of the precursor and the fully processed peptides indicates significant structural similarity. A 71 kDa form of the glutaminase was also observed when HTC cells were pulse-labelled for 2-6 min with [35S]methionine. Pulse-chase experiments indicate that the cytoplasmic precursor is quantitatively converted into the mature forms of the glutaminase. In addition, the observed kinetics established that the 71 kDa peptide is a true intermediate in the import of the mitochondrial glutaminase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behra R., Christen P. In vitro import into mitochondria of the precursor of mitochondrial aspartate aminotransferase. J Biol Chem. 1986 Jan 5;261(1):257–263. [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Clark V. M., Curthoys N. P. Cause of subunit heterogeneity in purified rat renal phosphate-dependent glutaminase. J Biol Chem. 1979 Jun 25;254(12):4939–4941. [PubMed] [Google Scholar]
- Clark V. M., Shapiro R. A., Curthoys N. P. Comparison of the hydrolysis and the covalent binding of 6-diazo-5-oxo-L-[6-14C]norleucine by rat renal phosphate-dependent glutaminase. Arch Biochem Biophys. 1982 Jan;213(1):232–239. doi: 10.1016/0003-9861(82)90457-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Curthoys N. P., Kuhlenschmidt T., Godfrey S. S., Weiss R. F. Phosphate-dependent glutaminase from rat kidney. Cause of increased activity in response to acidosis and identity with glutaminase from other tissues. Arch Biochem Biophys. 1976 Jan;172(1):162–167. doi: 10.1016/0003-9861(76)90062-x. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Lowry O. H. The distribution of glutaminase isoenzymes in the various structures of the nephron in normal, acidotic, and alkalotic rat kidney. J Biol Chem. 1973 Jan 10;248(1):162–168. [PubMed] [Google Scholar]
- Curthoys N. P., Weiss R. F. Regulation of renal ammoniagenesis. Subcellular localization of rat kidney glutaminase isoenzymes. J Biol Chem. 1974 May 25;249(10):3261–3266. [PubMed] [Google Scholar]
- Godfrey S., Kuhlenschmidt T., Curthoys P. Correlation between activation and dimer formation of rat renal phosphate-dependent glutaminase. J Biol Chem. 1977 Mar 25;252(6):1927–1931. [PubMed] [Google Scholar]
- Hartl F. U., Ostermann J., Guiard B., Neupert W. Successive translocation into and out of the mitochondrial matrix: targeting of proteins to the intermembrane space by a bipartite signal peptide. Cell. 1987 Dec 24;51(6):1027–1037. doi: 10.1016/0092-8674(87)90589-7. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Pfanner N., Nicholson D. W., Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989 Jan 18;988(1):1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Schmidt B., Wachter E., Weiss H., Neupert W. Transport into mitochondria and intramitochondrial sorting of the Fe/S protein of ubiquinol-cytochrome c reductase. Cell. 1986 Dec 26;47(6):939–951. doi: 10.1016/0092-8674(86)90809-3. [DOI] [PubMed] [Google Scholar]
- Haser W. G., Shapiro R. A., Curthoys N. P. Comparison of the phosphate-dependent glutaminase obtained from rat brain and kidney. Biochem J. 1985 Jul 15;229(2):399–408. doi: 10.1042/bj2290399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick J. P., Hodges P. E., Rosenberg L. E. Survey of amino-terminal proteolytic cleavage sites in mitochondrial precursor proteins: leader peptides cleaved by two matrix proteases share a three-amino acid motif. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4056–4060. doi: 10.1073/pnas.86.11.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaussi R., Sonderegger P., Flückiger J., Christen P. Biosynthesis and topogenesis of aspartate aminotransferase isoenzymes in chicken embryo fibroblasts. The precursor of the mitochondrial isoenzyme is either imported into mitochondria or degraded in the cytosol. J Biol Chem. 1982 Nov 25;257(22):13334–13340. [PubMed] [Google Scholar]
- Kalousek F., Hendrick J. P., Rosenberg L. E. Two mitochondrial matrix proteases act sequentially in the processing of mammalian matrix enzymes. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7536–7540. doi: 10.1073/pnas.85.20.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox W. E., Horowitz M. L., Friedell G. H. The proportionality of glutaminase content to growth rate and morphology of rat neoplasms. Cancer Res. 1969 Mar;29(3):669–680. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mori M., Morita T., Ikeda F., Amaya Y., Tatibana M., Cohen P. P. Synthesis, intracellular transport, and processing of the precursors for mitochondrial ornithine transcarbamylase and carbamoyl-phosphate synthetase I in isolated hepatocytes. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6056–6060. doi: 10.1073/pnas.78.10.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulation of renal ammoniagenesis. Purification and characterization of phosphate-dependent glutaminase from rat kidney. Arch Biochem Biophys. 1976 May;174(1):82–89. [PubMed] [Google Scholar]
- Reid G. A., Schatz G. Import of proteins into mitochondria. Extramitochondrial pools and post-translational import of mitochondrial protein precursors in vivo. J Biol Chem. 1982 Nov 10;257(21):13062–13067. [PubMed] [Google Scholar]
- Reid G. A., Yonetani T., Schatz G. Import of proteins into mitochondria. Import and maturation of the mitochondrial intermembrane space enzymes cytochrome b2 and cytochrome c peroxidase in intact yeast cells. J Biol Chem. 1982 Nov 10;257(21):13068–13074. [PubMed] [Google Scholar]
- Schatz G., Butow R. A. How are proteins imported into mitochondria? Cell. 1983 Feb;32(2):316–318. doi: 10.1016/0092-8674(83)90450-6. [DOI] [PubMed] [Google Scholar]
- Shapiro R. A., Banner C., Hwang J. J., Wenthold R. J., Curthoys N. P. Regulation of renal glutaminase gene expression during metabolic acidosis. Contrib Nephrol. 1988;63:141–146. doi: 10.1159/000415712. [DOI] [PubMed] [Google Scholar]
- Shapiro R. A., Clark V. M., Curthoys N. P. Covalent interaction of L-2-amino-4-oxo-5-chloropentanoic acid with rat renal phosphate-dependent glutaminase. Evidence for a specific glutamate binding site and of subunit heterogeneity. J Biol Chem. 1978 Oct 10;253(19):7086–7090. [PubMed] [Google Scholar]
- Shapiro R. A., Haser W. G., Curthoys N. P. Immunoblot analysis of glutaminase peptides in intact and solubilized mitochondria isolated from various rat tissues. Biochem J. 1987 Mar 15;242(3):743–747. doi: 10.1042/bj2420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R. A., Haser W. G., Curthoys N. P. The orientation of phosphate-dependent glutaminase on the inner membrane of rat renal mitochondria. Arch Biochem Biophys. 1985 Nov 15;243(1):1–7. doi: 10.1016/0003-9861(85)90767-2. [DOI] [PubMed] [Google Scholar]
- Skerjanc I. Mitochondrial import: properties of precursor proteins. Biochem Cell Biol. 1990 Jan;68(1):9–16. doi: 10.1139/o90-002. [DOI] [PubMed] [Google Scholar]
- Sztul E. S., Chu T. W., Strauss A. W., Rosenberg L. E. Import of the malate dehydrogenase precursor by mitochondria. Cleavage within leader peptide by matrix protease leads to formation of intermediate-sized form. J Biol Chem. 1988 Aug 25;263(24):12085–12091. [PubMed] [Google Scholar]
- Tannen R. L., Sastrasinh S. Response of ammonia metabolism to acute acidosis. Kidney Int. 1984 Jan;25(1):1–10. doi: 10.1038/ki.1984.1. [DOI] [PubMed] [Google Scholar]
- Tong J., Shapiro R. A., Curthoys N. P. Changes in the levels of translatable glutaminase mRNA during onset and recovery from metabolic acidosis. Biochemistry. 1987 May 19;26(10):2773–2777. doi: 10.1021/bi00384a018. [DOI] [PubMed] [Google Scholar]