Abstract
Numerous studies have linked folate deficiency and resultant elevated plasma homocysteine levels with an increased risk of neurodegenerative diseases and seizures. The aim of the present study was to examine the effect of acute folic acid administration on behavioural and electroencephalographic (EEG) characteristics of DL homocysteine thiolactone-induced seizures in adult rats. Adult male Wistar rats were divided into following groups: (1) saline-treated (C); (2) DL homocysteine thiolactone 8 mmol/kg, i.p. (H); (3) groups that received folic acid i.p. in doses: 5 mg/kg (F5), 10 mg/kg (F10) and 15 mg/kg (F15) and (4) F 30 min prior to H (F5H, F10H and F15H, respectively). Seizure behaviour was assessed by incidence, latency, number and intensity of seizure episodes. Seizure severity was described by a descriptive scale with grades 0–4. For EEG recordings, under pentobarbital anaesthesia three gold-plated recording electrodes were implanted into the skull. There were no behavioural and EEG signs of seizure activity in C, F5, F10 and F15 groups. In F15H group, the incidence of seizures was significantly lower, and the latency significantly prolonged, comparing to the H. Pre-treatment with F did not affect median number and severity of seizure episodes in all FH groups. Administration of F decreased mean total spectral power density in all FH groups, in a dose-dependent manner, in comparison with H. Our findings suggest that folic acid has anticonvulsive and antiepileptic effect on H-induced seizures in adult rats.
Keywords: Folic acid, DL homocysteine thiolactone, Seizures, Electroencephalography, Rats
Introduction
Folate is a water soluble vitamin of the B complex. Humans and other mammals cannot synthesize folate and, thus, must obtain the vitamin from nutritional sources via intestinal absorption. Natural dietary folates are chemically very labile and bioavailability is about 50%, whereas synthetic form is a chemically stable and almost 100% bioavailable form of folate (Djukic 2007).
A growing number of epidemiological studies have linked folate deficiency with an increased risk of vascular disease, cerebral ischemia, seizures, neurodegenerative and neuropsychiatric diseases, including Alzheimer’s and Parkinson’s disease (Mattson et al. 2002) depression and schizophrenia. In folate deficient medium, proliferation of neural progenitor cells is reduced, while folate deficiency in vivo decreases the number of proliferating cells in the dentate gyrus of the hippocampus in adult mice (Kruman et al. 2005), affects oligodendrocytes, astroglia and microglia (Bruce-Gregorios et al. 1991; Pak et al. 2003; Vairano et al. 2004). In humans, folate deficiency has been associated with reduced hippocampal and amygdalar volumes, atrophy of the cerebral cortex, global brain atrophy and neural tube defects (den Heijer et al. 2003; Scott et al. 2004; Yang et al. 2007; Blom et al. 2006).
The full range of mechanisms by which folate deficiency may contribute to neurodegeneration is unclear. However, one impact of folate deprivation is increases in homocysteine. Folate is required both in the remethylation of homocysteine to methionine and in the synthesis of S-adenosyl-methionine (SAM), the principal biological methyl donor in numerous methylation reactions. Furthermore, via its donation of a methyl group, folate plays a key role in a variety of physiologic processes such as: neurotransmitter synthesis, DNA biosynthesis, regulation of gene expression, amino acid synthesis and metabolism, and myelin synthesis and repair (Hamid et al. 2009).
Homocysteine was previously shown to elicit seizures in immature animals (Kubova et al. 1995; Folbergrova 1997). Available data suggest that homocysteine can be harmful to human cells because of its metabolic conversion to a reactive thioester homocysteine thiolactone. Homocysteine and its metabolites seem to express direct excitatory effects on N-methyl-d-aspartate (NMDA) and group I metabotropic glutamate receptors (mGluRs) (Lipton et al. 1997; Troen 2005). Recently, a model of generalized homocysteine thiolactone-induced seizures in adult rats has been developed, in which coexistence of convulsive and absence-like seizures, accompanied by characteristic spike-and-wave discharges (SWD) in electroencephalogram, were proven enabling further investigations of their mechanisms using convulsive and subconvulsive doses of homocysteine (Stanojlović et al. 2009). The model has also been shown suitable for testing potential anticonvulsive substances (Hrncic et al. 2011; Rašic-Markovic et al. 2009).
In view of these considerations, the aim of the present study was to examine the effect of acute folic acid administration on behavioural and electroencephalographic (EEG) characteristics of DL homocysteine thiolactone-induced seizures in adult rats.
Materials and Methods
Animals and Experimental Conditions
All experimental procedures were carried out in accordance with The European Council Directive (86/609/EEC) and were approved by the Animal Care Committee of the University of Belgrade (298/5-2).
Adult 2-month-old Wistar male rats (170–200 g), obtained from the Military Medical Academy Breeding Laboratories, Belgrade (Serbia), were used. The animals were housed individually in transparent plastic wire-covered cages (55 × 35 × 15 cm) with free access to food and water. They were kept in a sound-attenuated chamber under controlled ambient conditions (22–23°C, 50–60% relative humidity, 12/12 h light/dark cycle with light switched on at 8 a.m.), and all tests took place during light period. The acclimatization period lasted for 7 days.
Study Design and Experimental Procedures
The animals were divided into the following groups: (1) controls, saline-injected (C, 0.9% NaCl, n = 10); (2) DL homocysteine thiolactone 8.0 mmol/kg (H, n = 10); (3) folic acid in doses: 5 mg/kg (F5, n = 8), 10 mg/kg (F10, n = 10) and 15 mg/kg (F15, n = 8), and (4) folic acid (F5, F10, F15) 30 min prior to H: F5H (n = 10), F10H (n = 14) and F15H (n = 14).
Each rat was used only once. All the substances were freshly dissolved in saline and after adjusting the pH to 7.4, administered intraperitoneally (i.p.) in a volume of 0.1 ml/100 g rat body weight.
Behavioural Recordings
Behavioural manifestations of H-induced epilepsy in rats were recorded. The animals placed in separate transparent plastic cages (55 × 35 × 15 cm) were observed for 120 min for the occurrence of convulsive behaviour. This was assessed by the incidence of seizures (the number of convulsing animals out of total number of rats), a number of seizure episodes per rat and the seizure severity. Seizure severity was determined by a modified descriptive rating scale reported by Stanojlović et al. (2009) with grades defined as: grade 1—head nodding, lower jaw twitching; grade 2—myoclonic body jerks (hot plate reaction), bilateral forelimb clonus with full rearing (Kangaroo position); grade 3—progression to generalized clonic convulsions followed by tonic extension of fore- and hind limbs and tail and grade 4—prolonged severe tonic-clonic convulsions lasting over 10 s (status epilepticus) or frequent repeated episodes of clonic convulsions for an extended period of time (over 5 min). In addition, latency to seizure, defined as a time from H injection to the first seizure episode, was also recorded. For rats without seizures, 90 min latency time was scored. Lethality was recorded 90 min and 24 h after H administration.
Surgery and EEG Recordings
The rats were anesthetized with pentobarbital sodium (50 mg/kg, i.p.), placed in a stereotaxic apparatus, and three gold-plated recording electrodes were implanted over frontal (AP 2; L 2.5), parietal (AP −2; L 6) and occipital (AP −5; L 4) cortices. All coordinates are given in millimetre relative to the bregma. Animals were allowed at least 7 days recovery from the surgery and then acclimated to the recording environment for at least 24 h. During that period, animals were supervised for the occurrence of convulsive behaviour.
An 8-channel EEG apparatus (RIZ, Zagreb, Croatia) was used. The signals were digitized using a SCB-68 data acquisition card (National Instruments Co, Austin, TX). A sampling frequency of 512 Hz/channel and 16-bit A/D conversion was used for the EEG signals. The cut-off frequencies for EEG recordings were set at 0.3 and 80 Hz for the high-pass and low-pass filters, respectively. Ambient noise was eliminated using a 50 Hz notch filter. Data acquisition and signal processing were performed with LabVIEW software developed in the laboratory (NeuroSciLaBG).
All EEG recordings in freely moving rats were visually monitored and screened for seizure activity and stored on disk for subsequent off-line analysis. The observational period (120 min) was divided into eight 15 min intervals. From each interval, a 5-min period of EEG was chosen (near to the midpoint of the 15 min interval) during typical and characteristic vigilance state. After that, 20 consecutive 12-s epochs of EEG were extracted, and the mean total power spectrum density (PSD) was calculated. Baseline EEG was recorded for 30 min, and calculated PSD was displayed in the graph at 0 min point. The fast Fourier transform method (linear detrending, Hanning window, 0.083 Hz resolution) was applied to obtain estimates of total PSD (μV2/Hz). Epochs with artefacts and with a significant amount of contamination by electromyographic activity were carefully detected and excluded from the analysis.
Drugs
All drugs were of analytical purity and purchased from Sigma-Aldrich Chemical Co., USA.
Data Analyses
Significance of the differences in the incidence of seizures and lethality was evaluated by Fisher’s exact probability test. Since the normal distribution of the data on seizure latency, number and intensity of seizure episodes has not been estimated by Kolmogorov–Smirnov test, the non-parametric analyses (Kruskal–Wallis ANOVA and Mann–Whitney U test) were used to determine the statistical significance of the differences between the groups (*P < 0.05, **P < 0.01). The results were expressed as medians with 25th and 75th percentiles.
Differences in mean total PSD between the groups were compared with one-way ANOVA. For data not normally distributed, Kruskal–Wallis one-way ANOVA on Ranks and a Dunns post hoc analysis were applied.
Results
Seizure Behaviour
Animals in the C and F groups expressed the normal gross behavioural activity without any sign of seizures, and no lethality was recorded. Convulsions were observed in all rats that received DL homocysteine thiolactone (H) in the dose of 8 mmol/kg (H, incidence 100%).
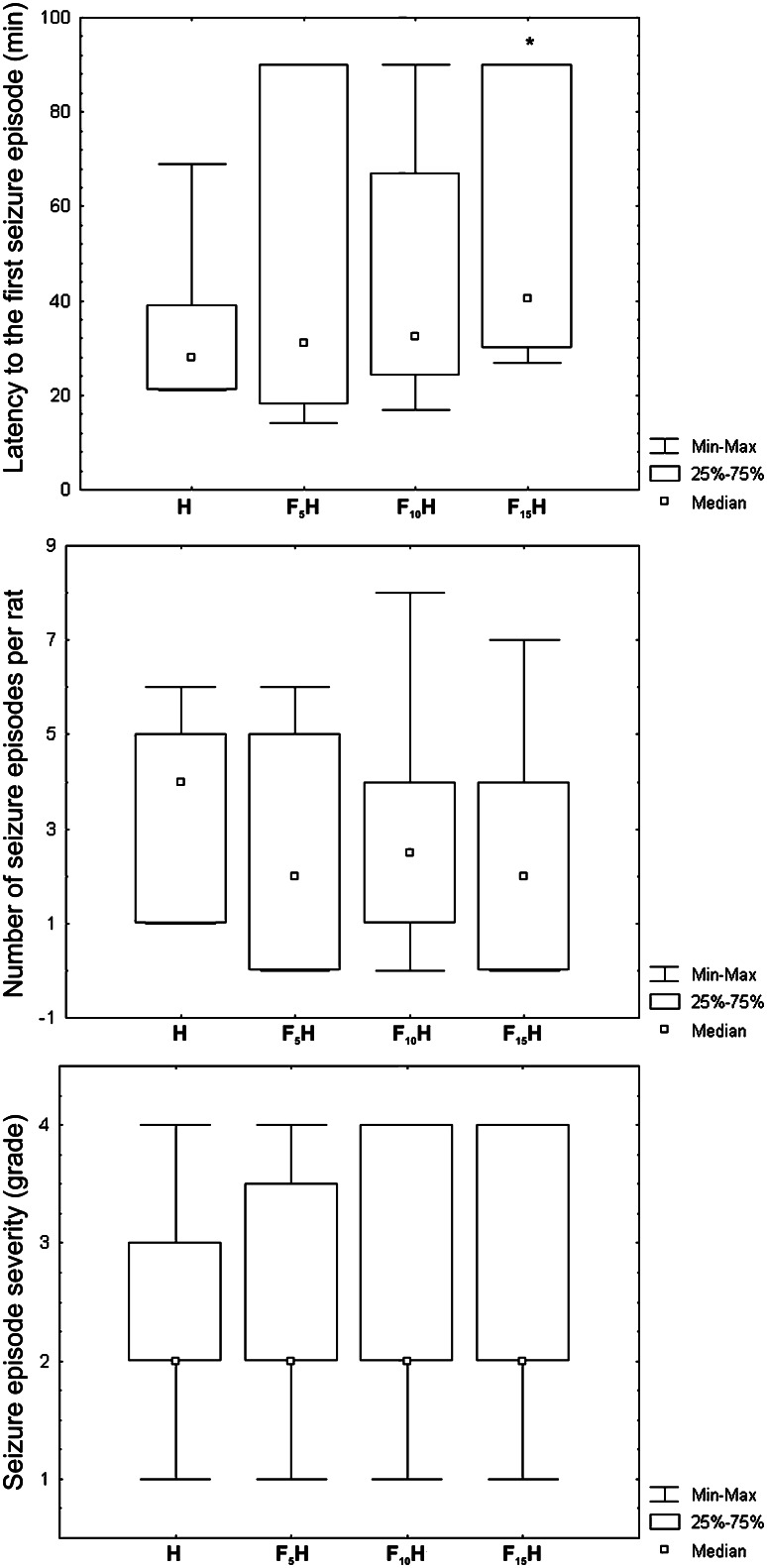
Folic acid administration significantly decreased seizure incidence in F15H (57.1%, P < 0.05) in comparison with H (100.0%) (Fig. 1). The highest dose of folic acid prolonged the median latency to the first seizure episode in F15H [40.5 (29.7–90.0) min] (P < 0.05), compared to the H [28.0 (21.0–39.0) min] (Fig. 2a).
Fig. 1.
The effect of acute folic acid administration on incidence of seizures induced by DL homocysteine thiolactone. The significance of the differences between the groups was estimated by Fisher’s exact probability test (*P < 0.05). Adult Wistar rats were i.p. treated with DL homocysteine thiolactone 8 mmol/kg (H) 30 min after folic acid 5, 10 and 15 mg/kg (F5H, F10H and F15H)
Fig. 2.
The effect of acute folic acid administration on median latency to the first seizure episode (a), a number of seizure episodes per rat (b) and seizure severity (c). The significance of the differences between the groups was estimated by Kruskal–Wallis ANOVA and Mann–Whitney U test (*P < 0.05 comparing to H). For the details see caption to Fig. 1
Acute folic acid administration did not affect the median number of seizure episodes per rat in experimental groups (Fig. 2b). Median seizure episode severity was not affected by folic acid administration (Fig. 2c). In order to examine the seizure severity, the statistical significance of seizure episode severity distribution was estimated by Fisher’s exact probability test. The majority of seizure episodes in all groups: H (40.0%), F5H (50.0%), F10H (48.8%) and F15H (56.3%) were of grade 2 (Table 1).
Table 1.
The effect of acute folic acid administration on seizure episode severity grade distribution in experimental groups
| Grade [%] | Experimental groups | |||
|---|---|---|---|---|
| H | F5H | F10H | F15H | |
| 1 | 16.0 | 4.2 | 9.3 | 0.0 |
| 2 | 40.0 | 50.0* | 48.8* | 56.3* |
| 3 | 24.0 | 20.8 | 14.0 | 18.8 |
| 4 | 20.0 | 25.0 | 27.9 | 25.0 |
Lethal outcomes 90 min 24 h upon H injection were not affected by folic acid administration (Table 2).
Table 2.
The effect of acute folic acid administration on lethality recorded 90 min and 24 h after DL homocysteine thiolactone administration
| Lethality [%] | Experimental groups | |||
|---|---|---|---|---|
| H | F5H | F10H | F15H | |
| After 90 min | 42.85 | 14.29 | 7.14 | 30.00 |
| After 24 h | 85.70 | 50.00 | 57.14 | 60.00 |
Lethality number of exited rats out of total number of rats in group expressed in percentage
Significance of the differences between the groups was estimated by Fisher’s exact probability test
For details, see caption of Fig. 1
EEG Analysis
Bioelectrical activity recorded from the frontal, parietal and occipital cortex in the groups of rats treated only folic acid (F5, F10 and F15) was similar to the one in control, revealing no epileptiform graphoelements, while PSD was dominant in the alpha frequency range. During recordings, the rats were quiet, but awake. The power spectra analysis showed that there were no significant differences in the mean total PSD between C and F groups (data not shown).
Bioelectrical activity demonstrated isolated spikes, classified as the first sign of H action, which progressed to the spike wave complexes and burst of spikes. Dissociation between EEG pattern and motor phenomena, as well as low electroclinical correlation, was common to all experimental recordings. EEG analyses have shown that epileptic attacks in FH groups had lower amplitude comparing to the H group. In H group, the convulsions of grade 2 were usually accompanied by bursts of polyspikes in EEG. Administration of folic acid decreased the amplitude of polyspikes in all FH groups. Convulsions in F15H were not followed by characteristic EEG pattern, furthermore, instead of burst of spikes characteristic to the grade 2 convulsions, isolated low amplitude spikes were recorded (Fig. 3).
Fig. 3.
Representative EEG tracings (the left panels) and corresponding mean total power spectra density (PSD, the right panels) in experimental groups, recorded during grade 2 convulsions, 45 min after DL homocysteine thiolactone administration. See the caption for Fig. 1
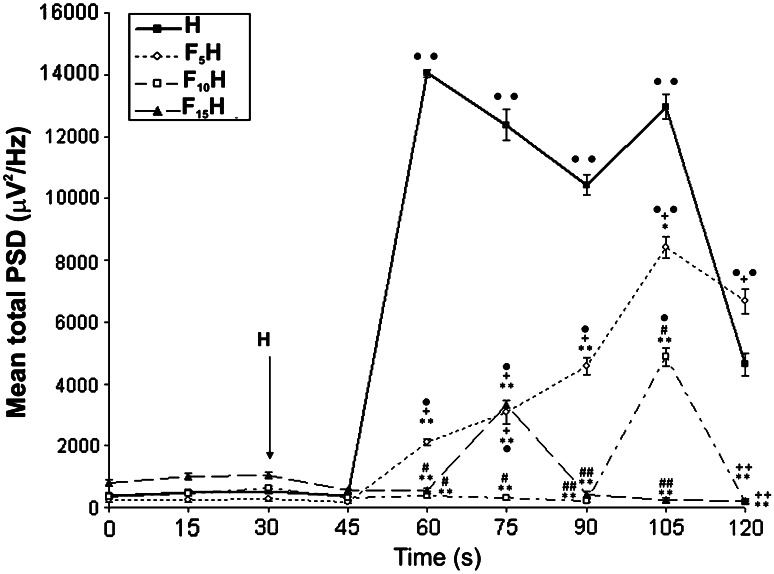
Application of folic acid 30 min before H significantly decreased mean total PSD in all FH groups (F5H, F10H and F15H), comparing to the H (Fig. 4) group. In 45–105 min period (30–70 min after H injection), PSD was significantly lower in F5H (P < 0.01), F10H (P < 0.01) and F15H (P < 0.01) in comparison with H (Fig. 4). In H and F5H groups, significant increase in mean total PSD, comparing to the basal conditions (P < 0.01 and P < 0.05, respectively), was recorded in 45–60 min period, and overlapped with latency to the first convulsive episode (28–31 min after H) in these groups. Although, the increase in PSD was recorded in both groups, the values recorded in F5H were significantly lower comparing to the H (P < 0.01). In 60–75 min period, total PSD in F15H significantly increased, comparing to the baseline (P < 0.05), and overlapped with the latency period in this group (Fig. 4). The peak of the PSD in F15H was recorded in the same period (60–75 min), and it was significantly lower in comparison with H (P < 0.01). In 90–105 min period, PSD in F10H increased significantly (P < 0.05) comparing to the baseline, and reached its maximum, which was significantly lower in comparison with H (P < 0.01) at same time point. Peak PSD in F5H was recorded at the same time point (90–105 min) and was significantly lower in comparison with H (P < 0.05), but when compared with F10H (P < 0.01) and F15H (P < 0.01) it was significantly higher. In 105–120 min period, mean total PSD in F10H and F15H returned to the baseline and were significantly lower comparing to the H (P < 0.01; P < 0.01) and F5H (P < 0.01; P < 0.01) groups (Fig. 4).
Fig. 4.
Time-course of EEG mean total PSD in consecutive 15 min periods in experimental groups. Statistical significance was estimated by one-way ANOVA. For data not normally distributed, Kruskal–Wallis one-way ANOVA ∙ P < 0.05; ∙∙ P < 0.01 versus baseline PSD; *P < 0.05; **P < 0.01 versus H; # P < 0.05; ## P < 0.01 versus F5H; + P < 0.05; ++ P < 0.01 versus F10H. For details, see caption of Fig. 1
Discussion
The link between epilepsy and folic acid is complex. Our results presented here demonstrate that mega dose of folic acid (15 mg/kg) when applied i.p. significantly decreases seizure incidence and prolongs the latency of the seizures induced by homocysteine. In our experiments, the highest dose of folic acid induced a modulation of ictal EEG patterns rather than a net change in total EEG activity: ictal high-amplitude polyspikes during grade 2 seizures were replaced by lower amplitude signals, and PSD returned to the baseline in 90 min period. Furthermore, EEG analyses have shown that acute folic acid administration in dose-dependent manner decreases mean total PSD and the amplitude of spikes during ictal period, in all FH groups.
Our findings are consistent with previous results regarding the role of folic acid in preventing and delaying neuronal death and excitotoxicity of retinal neurons induced by kainic acid, NMDA and quinolinic acid (Sattayasai and Ehrlich 1987) and cultured mouse cerebellar granule neurons induced by glutamate or NMDA (Lin et al. 2004). According to these results, it appears that folic acid is a potent antagonist of the NMDA and kainic receptors. Numerous authors have reported that folic acid prevents oxidative stress induced by homocysteine both in central nervous and cardiovascular system (Singh et al. 2011, Djuric et al. 2007). Folic acid prevents reduction of antioxidant enzymes: catalase, glutathione peroxidase and superoxide dismutase in brain of hyperhomocysteinemic rats (Singh et al. 2011, Matté et al. 2009), and decreases the level of malondialdehyde (Singh et al. 2011, Racek et al. 2005) and superoxide anion (Djuric et al. 2007). Finally, folic acid prevents inhibition of Na+/K+ ATPase induced by homocysteine administration (Matté et al. 2009, Wyse et al. 2002). EEG analysis results are in agreement with Baydar et al. (2002) who find out that folic acid supplementation do not affect electrocorticograms or evoked potentials. Furthermore folic acid promotes the regeneration of methionine from homocystein, lowers levels of homocysteine (Rydlewicz et al. 2002, Lamers et al. 2004) and attenuates excitotoxic effect of homocysteine.
Although multiple controlled studies (Norris and Pratt 1971, Gibberd et al. 1981) have failed to demonstrate any adverse effect on seizure frequency linked to the folic acid supplementation, some case reports (Guidolin et al. 1998; Raynolds and Wales 1960) have documented that a worsening of the seizure frequency may occur in some patients when folic acid is administered. Although a constant supply of folate is required for normal brain function, direct neurotoxic effects of excess folate have also been described (Olney et al. 1981; Weller et al. 1994). Intraventricular (Noell et al. 1960; Hommes and Obbens 1972; Hommes et al. 1973) or intracortical (Van Rijn et al. 1990) administration of folates induces convulsions; moreover, sodium folate is 100 times more epileptogenic than comparable non-molar concentrations of sodium glutamate (Hommes and Obbens 1972; Hommes et al. 1973). Furthermore, folic acid enhances the electrical kindling model of epilepsy (Miller et al. 1979) and the vitamin can even be used to kindle seizures directly (O’Donnell et al. 1983).
The discrepancy of our results and the reports of other authors may be ascribed to the differences in application route to animal. It is an important point that folic acid was applied i.p. in this study, while others directly injected folic acid into the brain (Noell et al. 1960; Hommes and Obbens 1972; Hommes et al. 1973; Van Rijn et al. 1990).
Data about the influence of folic acid on the CNS are conflicting and contradictory; on the one hand, folate has neuroprotective effect, while excess folate can exacerbate seizures in epileptics (Reynolds 2002). On the other hand, patients on antiepileptic drugs are prone to low folate and vitamin B12 and high total homocysteine concentrations of plasma (Apeland et al. 2002). Folate and its derivatives have diverse roles in the brain tissue: folate serves in the generation of glycine, glutamic acid and methionine; regulates homocysteine serum levels; it is important for the biosynthesis of serotonin, catecholamines and melatonin (Bottiglieri et al. 2000; Goff et al. 2004). There is strong evidence suggesting an acute antioxidant effect of this vitamin, which is independent of its effect on homocysteine metabolism (Ho et al. 2003, Racek et al. 2005, Ullegaddi et al. 2004) and it appears that folic acid is a potent antagonist of the NMDA and kainic receptors.
Further studies are necessary to clarify the mechanisms underlying anticonvulsive effect of folic acid and its relevance as adjuvant therapy in epileptic patients.
Acknowledgments
This work was supported by the Ministry of Science and Technological Development of Serbia, Grants No. 175032 and 175043.
References
- Apeland T, Mansoor MA, Pentieva K, McNulty H, Seljeflot I, Strandjord RE (2002) The effect of B-vitamins on hyperhomocysteinemia in patients on antiepileptic drugs. Epilepsy Res 51:237–247 [DOI] [PubMed] [Google Scholar]
- Baydar T, Papp A, Nagymajtényi L, Schulz H, Sahin G (2002) Folate supplementation in rats: does it cause behavioural and electrophysiological changes? Pteridines 13:107–114 [Google Scholar]
- Blom HJ, Shaw GM, den Heijer M, Finnell RH (2006) Neural tube defects and folate: case far from closed. Nat Rev Neurosci 7:724–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH (2000) Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry 69:228–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Gregorios JH, Agarwal RP, Oracion A, Ramirez A, Lin L (1991) Effects of methotrexate on RNA and purine synthesis of astrocytes in primary culture. J Neuropathol Exp Neurol 50:770–778 [DOI] [PubMed] [Google Scholar]
- den Heijer T, Vermeer SE, Clarke R, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM (2003) Homocysteine and brain atrophy on MRI of non-demented elderly. Brain 126:170–175 [DOI] [PubMed] [Google Scholar]
- Djukic A (2007) Folate-responsive neurologic diseases. Pediatr Neurol 37:387–397 [DOI] [PubMed] [Google Scholar]
- Djurić D, Vusanović A, Jakovljević V (2007) The effects of folic acid and nitric oxide synthase inhibition on coronary flow and oxidative stress markers in isolated rat heart. Mol Cell Biochem 300:177–183 [DOI] [PubMed] [Google Scholar]
- Folbergrova J (1997) Anticonvulsant action of both NMDA and non-NMDA receptor antagonists against seizures induced by homocysteine in immature rats. Exp Neurol 145:442–450 [DOI] [PubMed] [Google Scholar]
- Gibberd FB, Nicholls A, Wright MG (1981) The influence of folic acid on the frequency of epileptic attacks. Eur J Clin Pharmacol 19(1):57–60 [DOI] [PubMed] [Google Scholar]
- Goff DC, Bottiglieri T, Arning E, Shih V, Freudenreich O, Evins AE, Henderson DC, Baer L, Coyle J (2004) Folate, homocysteine, and negative symptoms in schizophrenia. Am J Psychiatry 161:1705–1708 [DOI] [PubMed] [Google Scholar]
- Guidolin L, Vignoli A, Canger R (1998) Worsening in seizure frequency and severity in relation to folic acid administration. Eur J Neurol 5:301–303 [DOI] [PubMed] [Google Scholar]
- Hamid A, Wani NA, Kaur J (2009) New perspectives on folate transport in relation to alcoholism-induced folate malabsorption—association with epigenome stability and cancer development. FEBS J 276:2175–2191 [DOI] [PubMed] [Google Scholar]
- Ho PI, Ashline D, Dhitavat S, Ortiz D, Collins SC, Shea TB, Rogers E (2003) Folate deprivation induces neurodegeneration: roles of oxidative stress and increased homocysteine. Neurobiol Dis 14:32–42 [DOI] [PubMed] [Google Scholar]
- Hommes OR, Obbens EA (1972) The epileptogenic action of Na-folate in the rat. J Neurol Sci 16:271–281 [DOI] [PubMed] [Google Scholar]
- Hommes OR, Obbens EA, Wijffels CC (1973) Epileptogenic activity of sodium-folate and the blood–brain barrier in the rat. J Neurol Sci 19:63–71 [DOI] [PubMed] [Google Scholar]
- Hrnčić D, Rašić-Marković A, Djuric D, Sušić V, Stanojlović O (2011) The role of nitric oxide in convulsions induced by lindane in rats. Food Chem Toxicol 49:947–954 [DOI] [PubMed] [Google Scholar]
- Kruman II, Mouton PR, Emokpae R Jr, Cutler RG, Mattson MP (2005) Folate deficiency inhibits proliferation of adult hippocampal progenitors. NeuroReport 16:1055–1059 [DOI] [PubMed] [Google Scholar]
- Kubova H, Folbergova J, Mareš P (1995) Seizures induced by homocysteine in rats during ontogenesis. Epilepsia 36:750–756 [DOI] [PubMed] [Google Scholar]
- Lamers Y, Prinz-Langenohl R, Moser R, Pietrzik K (2004) Supplementation with [6S]-5-methyltetrahydrofolate or folic acid equally reduces plasma total homocysteine concentrations in healthy women. Am J Clin Nutr 79:473–478 [DOI] [PubMed] [Google Scholar]
- Lin Y, Desbois A, Jiang S, Hou ST (2004) Group B vitamins protect murine cerebellar granule cells from glutamate/NMDA toxicity. NeuroReport 15:2241–2244 [DOI] [PubMed] [Google Scholar]
- Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudu PV, Arnelle DR, Stamler JS (1997) Neurotoxicity associated with dual actions of homocysteine at the N-methyl-d-aspartate receptor. Proc Natl Acad Sci USA 94:5923–5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matté C, Mackedanz V, Stefanello FM, Scherer EB, Andreazza AC, Zanotto C, Moro AM, Garcia SC, Gonçalves CA, Erdtmann B, Salvador M, Wyse AT (2009) Chronic hyperhomocysteinemia alters antioxidant defenses and increases DNA damage in brain and blood of rats: protective effect of folic acid. Neurochem Int 54:7–13 [DOI] [PubMed] [Google Scholar]
- Mattson MP, Kruman II, Duan W (2002) Folic acid and homocysteine in age-related disease. Ageing Res Rev 1:95–111 [DOI] [PubMed] [Google Scholar]
- Miller AA, Goff D, Webster RA (1979) Predisposition of laboratory animals to epileptogenic activity of folic acid. In: Botez MI, Reynolds EH (eds) Folic acid in neurology, psychiatry, and internal medicine. Raven Press, New York, pp 331–334 [Google Scholar]
- Noell MS, Magoss LH, Cohen LH (1960) Cerebral effects of folic acid, pyrimidines, amino acids and their antimetabolites. Electroencephalogr Clin Neurophysiol 12:238 [Google Scholar]
- Norris JW, Pratt RF (1971) A controlled study of folic acid in epilepsy. A controlled study of folic acid in epilepsy. Neurology 21(6):659–664 [DOI] [PubMed] [Google Scholar]
- O’Donnell RA, Leach MJ, Miller AA et al (1983) Folic acid induced kindling in rats: changes in brain amino acids. In: Blair JA (ed) Chemistry and biology of pteridines. Walter de Gruyter, Berlin, pp 801–825 [Google Scholar]
- Olney JW, Fuller TA, DeGubareff T (1981) Kainate-like neurotoxicity of folates. Nature 292:165–167 [DOI] [PubMed] [Google Scholar]
- Pak KJ, Chan SL, Mattson MP (2003) Homocysteine and folate deficiency sensitize oligodendrocytes to the cell death-promoting effects of a presenilin-1 mutation and amyloid beta-peptide. Neuromol Med 3:119–128 [DOI] [PubMed] [Google Scholar]
- Racek J, Rusnakova H, Trefil L, Siala KK (2005) The influence of folate and antioxidants on homocysteine levels and oxidative stress in patients with hyperlipidemia and hyperhomocysteinemia. Physiol Res 54:87–95 [DOI] [PubMed] [Google Scholar]
- Rašić-Marković A, Djuric D, Hrnčić D, Lončar-Stevanović H, Vučević D, Mladenović D, Brkić P, Djuro M, Stanojlović O (2009) High dose of ethanol decreases EEG total spectral power density in seizures induced by d,l-homocysteine thiolactone in adult rats. Gen Physiol Biophys 28 Spec No:25–32 [PubMed] [Google Scholar]
- Raynolds EH, Wales BM (1960) Effects of folic acid on the mental state and fit frequency of drug-treated epileptic patients. Lancet 1:1086–1088 [DOI] [PubMed] [Google Scholar]
- Reynolds EH (2002) Benefits and risks of folic acid to the nervous system. J Neurol Neurosurg Psychiatry 72:567–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydlewicz A, Simpson JA, Taylor RJ, Bond CM, Golden MHN (2002) The effect of folic acid supplementation on plasma homocyteine in an elderly population. Quart J Med 95:27–35 [DOI] [PubMed] [Google Scholar]
- Sattayasai J, Ehrlich D (1987) Folic acid protects chick retinal neurons against the neurotoxic action of excitatory amino acids. Exp Eye Res 44(4):523–535 [DOI] [PubMed] [Google Scholar]
- Scott TM, Tucker KL, Bhadelia A, Benjamin B, Patz S, Bhadelia R, Liebson E, Price LL, Griffith J, Rosenberg I, Folstein MF (2004) Homocysteine and B vitamins relate to brain volume and white-matter changes in geriatric patients with psychiatric disorders. Am J Geriatr Psychiatry 12:631–638 [DOI] [PubMed] [Google Scholar]
- Singh R, Kanwar SS, Sood PK, Nehru B (2011) Beneficial effects of folic acid on enhancement of memory and antioxidant status in aged rat brain. Cell Mol Neurobiol 31:83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanojlović O, Rasić-Marković A, Hrncić D, Susić V, Macut D, Radosavljević T, Djuric D (2009) Two types of seizures in homocysteine thiolactone-treated adult rats, behavioural and electroencephalographic study. Cell Mol Neurobiol 29:329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troen AM (2005) The central nervous system in animal models of hyperhomocysteinemia. Prog Neuropsychopharmacol Biol Psychiatry 29:1140–1151 [DOI] [PubMed] [Google Scholar]
- Ullegaddi R, Powers HJ, Gariballa S (2004) B-group vitamin supplementation mitigates oxidative damage after acute ischaemic stroke. Clin Sci 107:477–484 [DOI] [PubMed] [Google Scholar]
- Vairano M, Graziani G, Tentori L, Tringali G, Navarra P, Dello Russo C (2004) Primary cultures of microglial cells for testing toxicity of anticancer drugs. Toxicol Lett 148:91–94 [DOI] [PubMed] [Google Scholar]
- Van Rijn CM, Van der Velden TJ, Rodrigues de Miranda JF, Feenstra MG, Hiel JA, Hommes OR (1990) Folates: epileptogenic effects and enhancing effects on [3H]TBOB binding to the GABAA-receptor complex. Epilepsy Res 5:199–208 [DOI] [PubMed] [Google Scholar]
- Weller M, Marini AM, Martin B, Paul SM (1994) The reduced unsubstituted pteroate moiety is required for folate toxicity of cultured cerebellar granule neurons. J Pharmacol Exp Ther 269:393–401 [PubMed] [Google Scholar]
- Wyse AT, Zugno AI, Streck EL, Matté C, Calcagnotto T, Wannmacher CM, Wajner M (2002) Inhibition of Na(+), K(+)-ATPase activity in hippocampus of rats subjected to acute administration of homocysteine is prevented by vitamins E and C treatment. Neurochem Res 27:1685–1689 [DOI] [PubMed] [Google Scholar]
- Yang LK, Wong KC, Wu MY, Liao SL, Kuo CS, Huang RF (2007) Correlations between folate, B12, homocysteine levels, and radiological markers of neuropathology in elderly post-stroke patients. Am Coll Nutr 26:272–278 [DOI] [PubMed] [Google Scholar]