Abstract
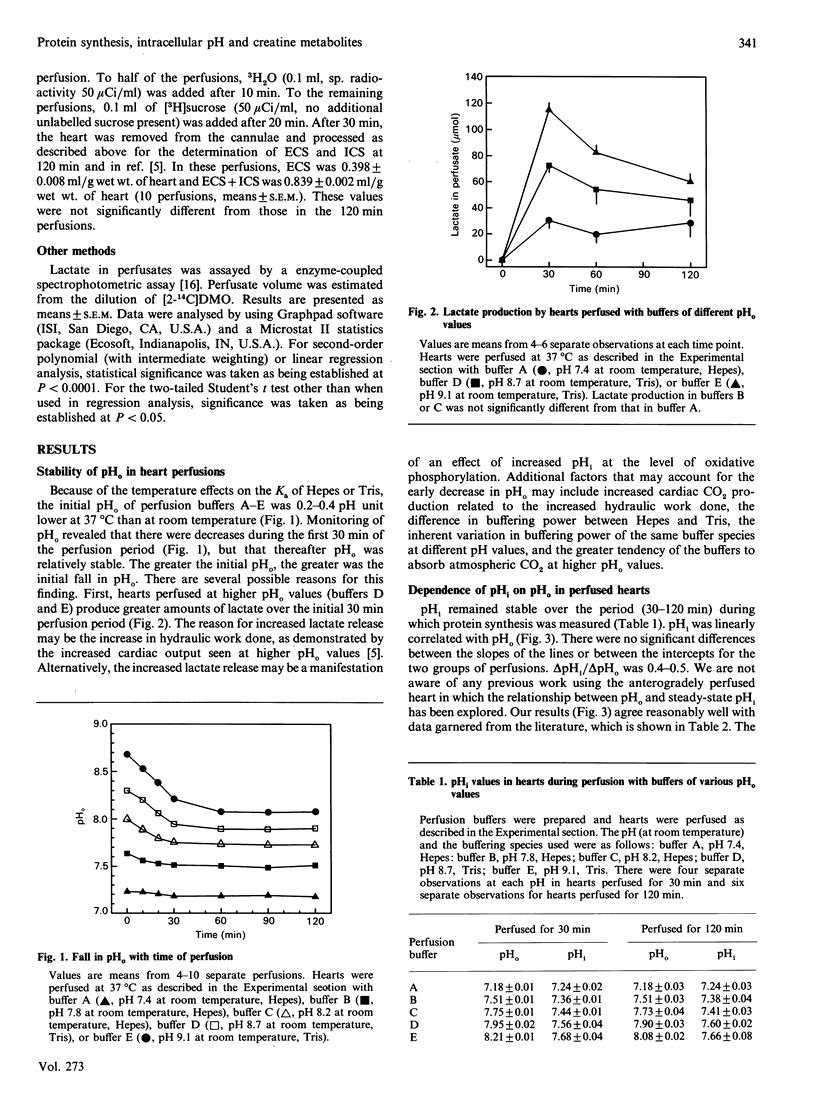
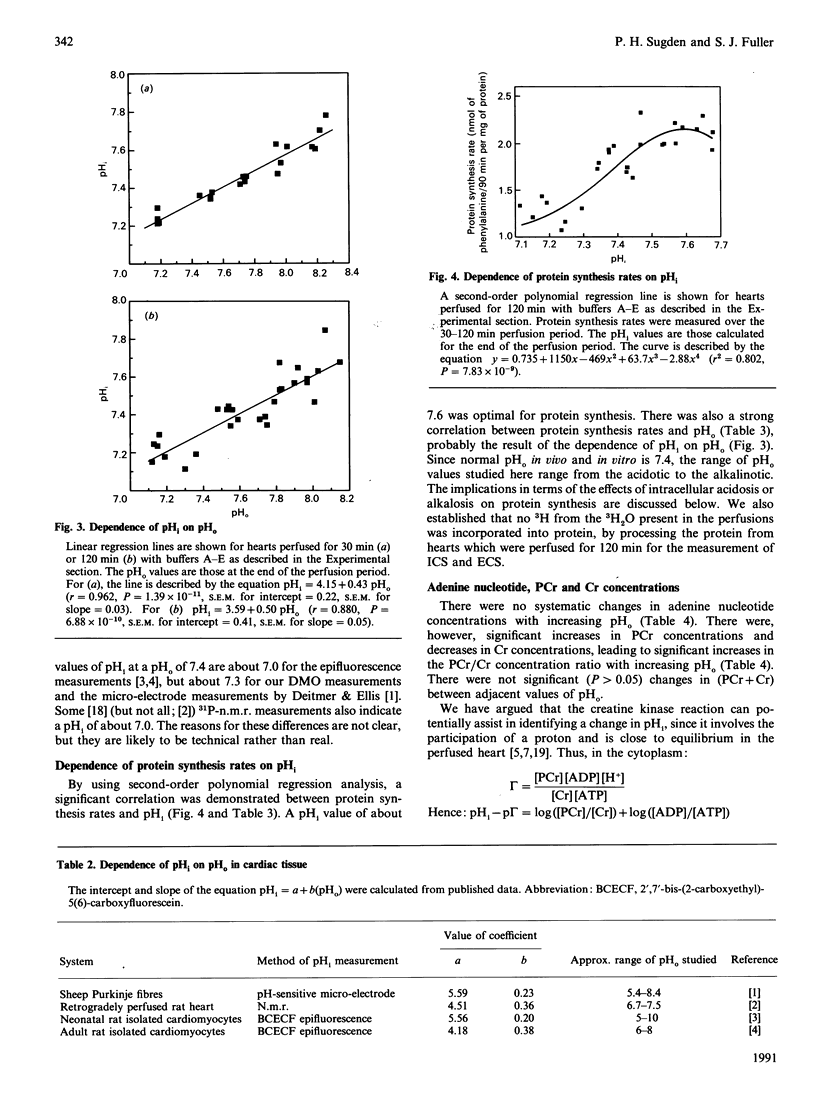
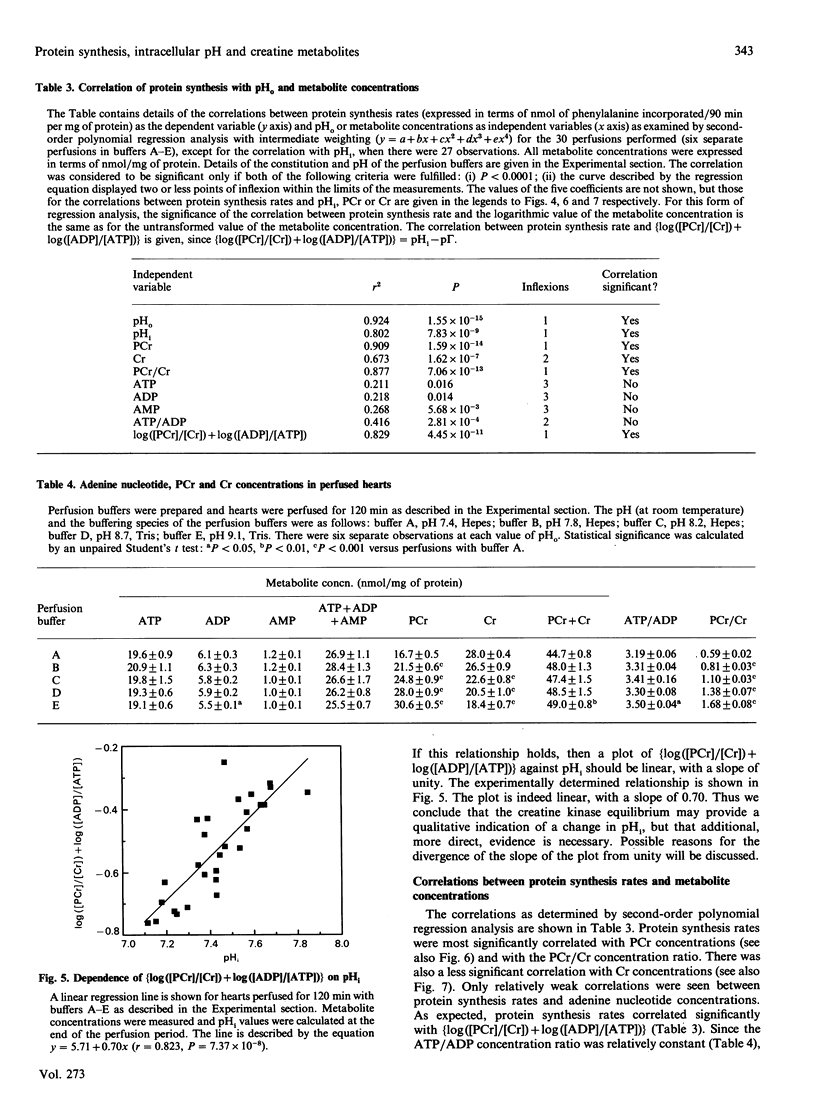
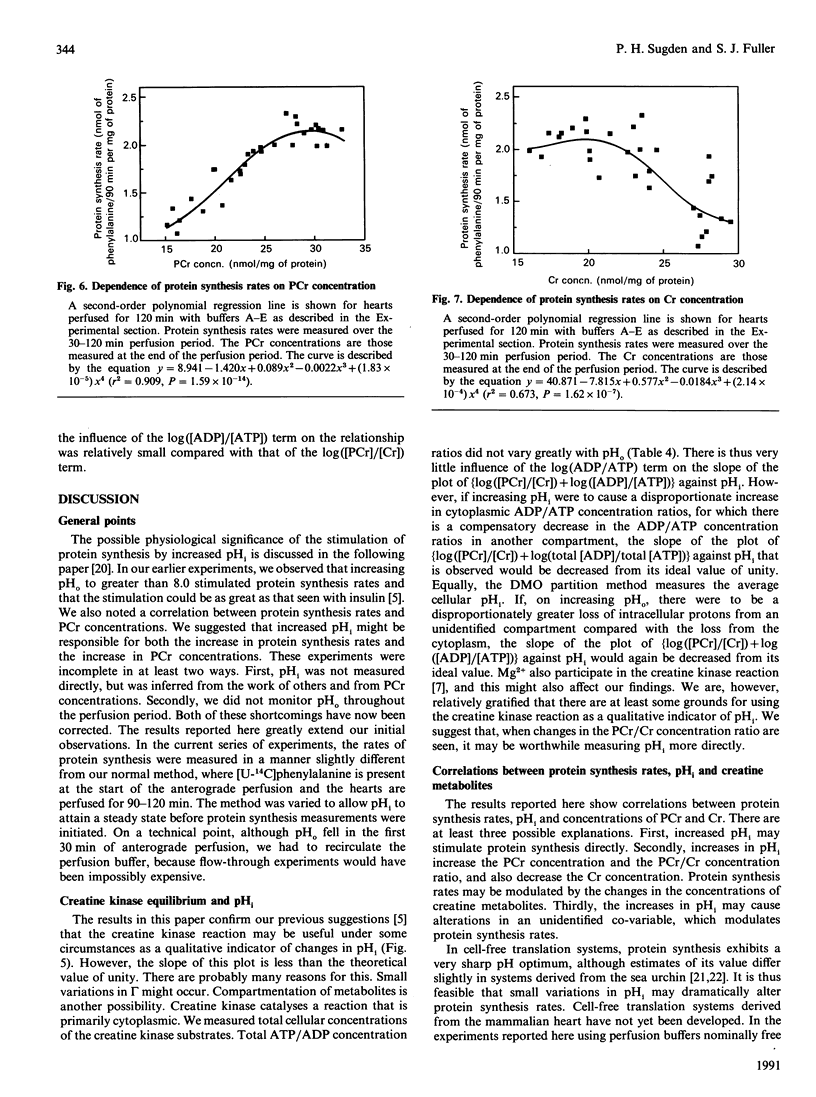
We have examined in detail the correlations between protein synthesis rates, intracellular pH (pHi) and the concentrations of creatine metabolites in the rat heart perfused anterogradely in vitro. Using perfusion buffers ranging from pH 7.2 to 8.2 at 37 degrees C, we were able to manipulate pHi from between 7.24 to 7.66, i.e. from the slightly acidotic to the alkalinotic as compared with the physiological values of pHi (about pH 7.29). The dependence of pHi on extracellular pH (pHo) was linear, with the value of delta pHi/delta pHo being 0.4-0.5. Protein synthesis rates were significantly stimulated when pHi was increased above its physiological value, and they were strongly correlated with pHi. They were also strongly correlated with phosphocreatine concentrations (and with creatine concentrations and phosphocreatine/creatine concentration ratios). Adenine nucleotide (ATP, ADP and AMP) concentrations and the ATP/ADP concentration ratio were not systematically altered by manipulating pHi, and protein synthesis rates showed only a relatively weak dependence on these variables. Since creatine kinase catalyses a reaction that is close to equilibrium in the perfused heart, and since phosphorylation of creatine involves release of a proton, we argue that the changes in phosphocreatine and creatine concentrations are manifestations of alterations in pHi. In this regard, we show that [log[( phosphocreatine]/[creatine]) + log [( ADP]/[ATP])] [the value of which gives [pHi--log (mass action ratio)]] is positively correlated with pHi, although the slope of the line is 0.7, as opposed to the ideal value of unity. We discuss three hypotheses to account for our observations: (i) protein synthesis rates are influenced directly by pHi, (ii) pHi affects the concentrations of creatine metabolites, which in turn affect protein synthesis rates, and (iii) pHi affects the value of an unidentified co-variable, which in turn affects protein synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bittl J. A., Ingwall J. S. Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. J Biol Chem. 1985 Mar 25;260(6):3512–3517. [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Interactions between the regulation of the intracellular pH and sodium activity of sheep cardiac Purkinje fibres. J Physiol. 1980 Jul;304:471–488. doi: 10.1113/jphysiol.1980.sp013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. J., Gaitanaki C. J., Hatchett R. J., 4th, Sugden P. H. Acute alpha 1-adrenergic stimulation of cardiac protein synthesis may involve increased intracellular pH and protein kinase activity. Biochem J. 1991 Jan 15;273(Pt 2):347–353. doi: 10.1042/bj2730347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. J., Gaitanaki C. J., Sugden P. H. Effects of catecholamines on protein synthesis in cardiac myocytes and perfused hearts isolated from adult rats. Stimulation of translation is mediated through the alpha 1-adrenoceptor. Biochem J. 1990 Mar 15;266(3):727–736. doi: 10.1042/bj2660727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. J., Gaitanaki C. J., Sugden P. H. Effects of increasing extracellular pH on protein synthesis and protein degradation in the perfused working rat heart. Biochem J. 1989 Apr 1;259(1):173–179. doi: 10.1042/bj2590173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. J., Sugden P. H. Acute inhibition of rat heart protein synthesis in vitro during beta-adrenergic stimulation or hypoxia. Am J Physiol. 1988 Oct;255(4 Pt 1):E537–E547. doi: 10.1152/ajpendo.1988.255.4.E537. [DOI] [PubMed] [Google Scholar]
- Gaitanaki C. J., Sugden P. H., Fuller S. J. Stimulation of protein synthesis by raised extracellular pH in cardiac myocytes and perfused hearts. FEBS Lett. 1990 Jan 15;260(1):42–44. doi: 10.1016/0014-5793(90)80061-m. [DOI] [PubMed] [Google Scholar]
- Garlick P. B., Radda G. K., Seeley P. J. Studies of acidosis in the ischaemic heart by phosphorus nuclear magnetic resonance. Biochem J. 1979 Dec 15;184(3):547–554. doi: 10.1042/bj1840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneda T., Watson P. A., Morgan H. E. Elevated aortic pressure, calcium uptake, and protein synthesis in rat heart. J Mol Cell Cardiol. 1989 Feb;21 (Suppl 1):131–138. doi: 10.1016/0022-2828(89)90848-1. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Pores I. H., Lucas S. K., Weisfeldt M. L., Flaherty J. T. Intracellular acidosis and contractility in the normal and ischemic heart as examined by 31P NMR. J Mol Cell Cardiol. 1982 Sep;14 (Suppl 3):13–20. doi: 10.1016/0022-2828(82)90124-9. [DOI] [PubMed] [Google Scholar]
- Kent R. L., Hoober J. K., Cooper G., 4th Load responsiveness of protein synthesis in adult mammalian myocardium: role of cardiac deformation linked to sodium influx. Circ Res. 1989 Jan;64(1):74–85. doi: 10.1161/01.res.64.1.74. [DOI] [PubMed] [Google Scholar]
- Kim D., Smith T. W. Altered Ca fluxes and contractile state during pH changes in cultured heart cells. Am J Physiol. 1987 Jul;253(1 Pt 1):C137–C146. doi: 10.1152/ajpcell.1987.253.1.C137. [DOI] [PubMed] [Google Scholar]
- Kim D., Smith T. W. Cellular mechanisms underlying calcium-proton interactions in cultured chick ventricular cells. J Physiol. 1988 Apr;398:391–410. doi: 10.1113/jphysiol.1988.sp017049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson J. W., Veech R. L. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979 Jul 25;254(14):6528–6537. [PubMed] [Google Scholar]
- Lopo A. C., Lashbrook C. C., Hershey J. W. Characterization of translation systems in vitro from three developmental stages of Strongylocentrotus purpuratus. Biochem J. 1989 Mar 1;258(2):553–561. doi: 10.1042/bj2580553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H. Effects of growth factors on intracellular pH regulation. Annu Rev Physiol. 1986;48:363–376. doi: 10.1146/annurev.ph.48.030186.002051. [DOI] [PubMed] [Google Scholar]
- Rubin I. B., Goldstein G. An ultrasensitive isotope dilution method for the determination of L-amino acids. Anal Biochem. 1970 Feb;33(2):244–254. doi: 10.1016/0003-2697(70)90293-9. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Fuller S. J., Sugden P. H. The effects of lactate, acetate, glucose, insulin, starvation and alloxan-diabetes on protein synthesis in perfused rat hearts. Biochem J. 1986 Jun 1;236(2):543–547. doi: 10.1042/bj2360543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. M., Sugden P. H. Effects of pressure overload and insulin on protein turnover in the perfused rat heart. Prostaglandins are not involved although their synthesis is stimulated by insulin. Biochem J. 1987 Apr 15;243(2):473–479. doi: 10.1042/bj2430473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. M., Sugden P. H. Stimulation of left-atrial protein-synthesis rates by increased left-atrial filling pressures in the perfused working rat heart in vitro. Biochem J. 1983 Dec 15;216(3):537–542. doi: 10.1042/bj2160537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Smith D. M. The effects of glucose, acetate, lactate and insulin on protein degradation in the perfused rat heart. Biochem J. 1982 Sep 15;206(3):467–472. doi: 10.1042/bj2060467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Smith D. M. The effects of insulin on glucose uptake and lactate release in perfused working rat heart preparations. Biochem J. 1982 Sep 15;206(3):473–479. doi: 10.1042/bj2060473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taegtmeyer H., Hems R., Krebs H. A. Utilization of energy-providing substrates in the isolated working rat heart. Biochem J. 1980 Mar 15;186(3):701–711. doi: 10.1042/bj1860701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Wallert M. A., Fröhlich O. Na+-H+ exchange in isolated myocytes from adult rat heart. Am J Physiol. 1989 Aug;257(2 Pt 1):C207–C213. doi: 10.1152/ajpcell.1989.257.2.C207. [DOI] [PubMed] [Google Scholar]


