Abstract
Human herpesvirus-8 (HHV-8) or Kaposi's sarcoma-associated herpesvirus K8.1 gene encodes for two immunogenic glycoproteins, gpK8.1A and gpK8.1B, originating from spliced messages. The 228-amino-acid (aa) gpK8.1A is the predominant form associated with the virion envelope, consisting of a 167-aa region identical to gpK8.1B and a 61-aa unique region (L. Zhu, V. Puri, and B. Chandran, Virology 262:237–249, 1999). HHV-8 has a broad in vivo and in vitro cellular tropism, and our studies showed that this may be in part due to HHV-8's interaction with the ubiquitous host cell surface molecule, heparan sulfate (HS). Since HHV-8 K8.1 gene is positionally colinear to the Epstein-Barr virus (EBV) gene encoding the gp350/gp220 protein involved in EBV binding to the target cells, gpK8.1A's ability to interact with the target cells was examined. The gpK8.1A without the transmembrane and carboxyl domains (ΔTMgpK8.1A) was expressed in a baculovirus system and purified. Radiolabeled purified ΔTMgpK8.1A protein bound to the target cells, which was blocked by unlabeled ΔTMgpK8.1A. Unlabeled ΔTMgpK8.1A blocked the binding of [3H]thymidine-labeled purified HHV-8 to the target cells. Binding of radiolabeled ΔTMgpK8.1A to the target cells was inhibited in a dose-dependent manner by soluble heparin, a glycosaminoglycan (GAG) closely related to HS, but not by other GAGs such as chondroitin sulfate A and C, N-acetyl heparin and de-N-sulfated heparin. Cell surface absorbed ΔTMgpK8.1A was displaced by soluble heparin. Radiolabeled ΔTMgpK8.1A also bound to HS expressing Chinese hamster ovary (CHO-K1) cells, and binding to mutant CHO cell lines deficient in HS was significantly reduced. The ΔTMgpK8.1A specifically bound to heparin-agarose beads, which was inhibited by HS and heparin, but not by other GAGs. Virion envelope-associated gpK8.1A was specifically precipitated by heparin-agarose beads. These findings suggest that gpK8.1A interaction with target cells involves cell surface HS-like moieties, and HHV-8 interaction with HS could be in part mediated by virion envelope-associated gpK8.1A.
Human herpesvirus 8 (HHV-8) or Kaposi's sarcoma-associated herpesvirus (KSHV) DNA has been detected in the Kaposi's sarcoma (KS) tissues from patients with AIDS-KS, classic KS, Africa-endemic KS, and transplantation-associated KS (12, 44). HHV-8 has a broad in vivo and in vitro cellular tropism. HHV-8 DNA and transcripts have been identified in vivo in human B cells (15), macrophages (4), endothelial cells (5, 52), and epithelial cells (16). In the KS tissues, HHV-8 DNA is present in a latent form in the vascular endothelial and spindle cells (5, 15, 17, 44, 52). In addition, a low percentage of HHV-8 lytic cycle has been detected in the infiltrating inflammatory monocytes (4). HHV-8 DNA has been also detected in primary effusion lymphomas or body-cavity-based lymphomas (BCBL) (9, 17). BCBL cell lines such as BCBL-1 and BC-3 carry HHV-8 in a latent form, and a lytic cycle can be induced by 12-O-tetradecanoylphorbol-13-acetate (TPA) (3, 17, 27, 40, 44, 50).
The in vitro infectious process of HHV-8 differ from many members of alpha-, beta-, and gammaherpesvirus families. HHV-8 has been shown to infect a variety of human and animal cells, such as human B cells, epithelial cells (293), human endothelial cells, human foreskin fibroblast (HFF) cells, human carcinoma cells (bladder, prostate, lung, and squamous), owl monkey kidney cells, and baby hamster kidney (BHK-21) cells (20, 34, 39, 58). If in vitro permissiveness of a cell type is judged by a productive lytic replication of HHV-8 after entry into cells, there is as yet no suitable cell culture system to support a lytic replication of input HHV-8. Only a latent HHV-8 infection is observed in the infected cells (20, 34, 39, 58). However, if in vitro permissiveness is judged by the establishment of HHV-8 latency and the ability to support HHV-8 lytic replication after activation by agents, cells such as HFF, human carcinoma cells and endothelial cells are permissive, as evidenced by the retention of viral genome in a latent form, by the expression of HHV-8 latency-associated open reading frame (ORF) 73 protein and by the ability to support lytic replication upon activation by agents such as TPA or by human cytomegalovirus (HCMV) infection (20, 34, 39, 58).
Since the analysis of in vitro HHV-8 interaction with host cells and quantitation of infection has been hampered by the absence of the lytic replication cycle and a reliable plaque assay, to monitor the HHV-8 binding and entry process BCBL-1 cells carrying a recombinant HHV-8 expressing the green fluorescent protein (GFP–HHV-8) were established (58). In a recent study, using the GFP–HHV-8 in the supernatant of TPA induced BCBL-GFP cells as the inoculum for infection and the [3H]thymidine-labeled purified HHV-8, we demonstrated that the broad cellular tropism of HHV-8 may be in part due to its interaction with the ubiquitous host cell surface HS molecule (2). This conclusion was based on the following findings: (i) HHV-8 infection of HFF cells was inhibited in a dose-dependent manner by soluble heparin, a glycosaminoglycan closely related to HS; (ii) enzymatic removal of HFF cell surface HS with heparinase I and III reduced HHV-8 infection; (iii) soluble heparin inhibited the binding of radiolabeled HHV-8 to human B-cell lines, embryonic kidney epithelial (293) cells, and HFF cells, suggesting interference at the virus attachment stage; (iv) cell surface-adsorbed HHV-8 was displaced by soluble heparin; and (v) radiolabeled HHV-8 also bound to wild-type HS expressing Chinese hamster ovary (CHO-K1) cells. In contrast, binding of virus to mutant CHO cells deficient in HS was significantly reduced. These data suggested that the gamma-2-HHV-8 is adsorbed to cells by binding to cell surface HS-like moieties. In this respect, gamma-2-HHV-8 resembles some members of the alpha (herpes simplex virus type 1 [HSV-1], HSV-2, pseudorabies virus [PRV], bovine herpesvirus 1 [BHV-1])-, beta (HCMV, HHV-7)-, and gamma-2 (BHV-4)-herpesviruses, where the initial virus-cell interaction also involves the binding to the cell surface HS (21, 24, 30, 31, 33, 35, 37, 46, 47, 49, 51, 57).
The identity of HHV-8 envelope glycoprotein(s) involved in the interaction with HS is not known. HHV-8 encodes for more than 80 ORFs, and ORFs 4 to 75 are designated based on the similarity to herpesvirus saimiri (HVS) ORFs (1). HHV-8 unique ORFs are designated with the prefix K (36, 43). HHV-8 has counterparts to other herpesvirus glycoproteins such as gB (ORF 8), gH (ORF 22), gM (ORF 39), and gL (ORF47) (36, 43). In addition, K1 and K8.1 genes encode for glycoproteins unique for HHV-8 (10, 36, 43). We have previously reported the identification of cDNAs originating from the HHV-8 K8.1 gene encoding two ORFs from spliced messages (10). One cDNA encoded for a 228-amino-acid (aa) protein designated gpK8.1.A and contains a signal sequence, transmembrane domain, and four N-glycosylation sites. The splicing event has generated the transmembrane domain in the gpK8.1A ORF not seen in the genomic K8.1 ORF. Another cDNA encoded for an ORF of 167 aa, designated gpK8.1.B. This protein has three N-glycosylation sites and shares similar amino and carboxy termini with ORF K8.1.A but with an in-frame deletion (10). Our studies with human sera demonstrated the immunogenic nature of gpK8.1A and gpK8.1B (10, 61). Using monoclonal antibodies (MAbs), we have also shown that gpK8.1A and gpK8.1B contain N- and O-linked sugars and that gpK8.1A is the predominant form detected within the infected cells and the virion envelopes (60).
HHV-8 K8.1 gene is positionally colinear to the glycoprotein genes in the members of gammaherpesvirus group such as the Epstein-Barr virus (EBV) gene encoding the major envelope glycoproteins gp350 and gp220 (22), gp150 of murine gammaherpesvirus 68 (MHV-68) (53), HVS ORF 51 gene (1), and the BORFD1 gene of BHV-4 (36, 43). EBV gp350/gp220 glycoprotein has been studied extensively and shown to be involved in the binding of the virus to the target cells (22). HHV-8 gpK8.1A shows several similarities to the EBV glycoproteins. Like EBV gp350/gp220, HHV-8 gpK8.1A elicits a strong human humoral immune response (61) and is a virion envelope-and infected cell membrane-associated glycoprotein containing both N- and O-linked sugars. Because of these similarities to EBV gp350/gp220, we examined the ability of HHV-8 gpK8.1A to interact with the target cells. We expressed gpK8.1A without the transmembrane and carboxyl domains (ΔTMgpK8.1A) in the baculovirus system and purified the protein. Using radiolabeled purified ΔTMgpK8.1A, we show that gpK8.1A interaction with target cells involves cell surface HS-like moieties. These results suggest that HHV-8 interaction with HS could be in part mediated by virion envelope gpK8.1A.
MATERIALS AND METHODS
Cells.
HFF cells, 293 cells, CHO-K1 cells (ATCC CCL-61), HS-deficient CHO derivative pgsD-677 cells (ATCC CRL-2244), HS-and chondroitin sulfate-deficient CHO derivative pgsA-745 cells (ATCC CRL-2242) (18, 32), BCBL-1 cells (HHV-8+ human B cells) (40, 50), and BJAB cells (HHV-8− human B cells) were used in this study. HFF and 293 monolayer cells were grown in Dulbecco modified Eagle medium (DMEM; Gibco-BRL, Grand Island, N.Y.) supplemented with 2 mM glutamine, 10% fetal bovine serum (FBS), and antibiotics. Suspension cultures of 293 cells were grown in 293-SF medium (Gibco-BRL). Adult human dermal microvascular endothelial cells (HMVEC-d Ad; CC-2543; Clonetics, San Diego, Calif.) were grown in endothelial growth medium (EGM; CC-3202; Clonetics). Monolayers of CHO-K1, pgsD-677, and pgsA-745 cells were grown in Ham's F-12K medium (Gibco-BRL) supplemented with 10% FBS and antibiotics. Suspension cultures of BCBL-1 and BJAB cells were grown in RPMI 1640 medium with glutaMAX I (Gibco-BRL) supplemented with 10% FBS and antibiotics. Spodoptera frugiperda ovarian cells (Sf9) were grown in TNM-FH insect medium (PharMingen, San Diego, Calif.).
Antibodies.
The production and characterization of MAbs against gpK8.1A and ORF 59 have been described previously (11, 60). High-titer-antibody-containing ascitic fluids were made by injecting hybridoma cells intraperitoneally into pristane-primed BALB/c mice. Immunoglobulin G (IgG) antibodies from the ascitic fluid and normal mouse sera were purified on protein A-Sepharose columns (Amersham Pharmacia Biotech AB, Uppsala, Sweden). Protein concentrations were adjusted to 1 mg/ml with phosphate-buffered saline (PBS; pH 7.4), and aliquots were stored at −20°C. Rabbit polyclonal antibodies raised against the baculovirus-expressed purified glutathione S-transferase–HHV-8 latency-associated ORF 73 protein (34, 58, 61) were used as a control.
Construction, expression, and purification of recombinant HHV-8 ΔTMgpK8.1A.
The 576-bp ΔTMgpK8.1A gene region encoding aa 1 to 192 lacking the transmembrane and the carboxyl domains was amplified from the full-length HHV-8 gpK8.1A cDNA (10) using the primers ΔTMgpK8.1A forward (5′-TTC CGC GTG AGC TCA TGA GTT CCA CAC AGA-3′ with a SacI site) and ΔTMgpK8.1A reverse (5′-GAT GGG TCG GTA CCT CTG CAT TGT AGT-3′ with a KpnI site) (Advantage cDNA PCR Kit; Clontech, Palo Alto, Calif.). The ΔTMgpK8.1A PCR product was cloned into the pAcHLT-A baculovirus transfer vector (PharMingen) and verified by sequencing. To generate the recombinant baculovirus, ΔTMgpK8.1A-pAcHLT-A plasmid was cotransfected with BaculoGold DNA (PharMingen) into Sf9 insect cells. Recombinant viruses were passaged three times before use.
His-tagged ΔTMgpK8.1A was expressed in Sf9 cells and purified using nickel columns (PharMingen) according to the manufacturer's recommendations. Briefly, Sf9 cells were infected with ΔTMgpK8.1A-baculovirus and, at 2 days postinfection, the cells were labeled with [35S]methionine for 20 h. Cell pellets were lysed with lysis buffer (10 mM Tris, pH 7.5; 130 mM NaCl; 1% Triton X-100; 10 mM NaF; 10 mM sodium orthophosphate; 10 mM sodium pyrophosphate), and centrifuged at 40,000 × g for 45 min at 4°C, and the clear supernatant was passed through an Ni-nitrilotriacetic acid-agarose column. The column was washed extensively with lysis buffer, followed by lysis buffer with 20 and 30 mM imidazole. Washes were monitored by measuring the optical density at 280 nm. When the A280 reached a value of <0.01, column-bound protein was eluted with 0.1 to 0.5 M imidazole and collected in 0.5-ml fractions. The purity of the eluted protein was analyzed by silver staining of sodium dodecyl sulfate (SDS)–12% polyacrylamide gel electrophoresis (PAGE) gels, by Western blots with anti-gpK8.1A MAb, and by autoradiography (60). Fractions containing the purified protein were pooled, dialyzed against PBS, concentrated, and reanalyzed as described above. His-tagged HHV-8 latency-associated ORF 73 protein (61) used as control was purified from Sf9 cells infected with ORF 73-pAcHLT-A baculovirus as described above.
Western blot assays.
Samples were boiled in sample buffer with 2-mercaptoethanol (2-ME), subjected to SDS-PAGE, and electrophoretically transferred onto nitrocellulose membranes. Standard prestained molecular weight markers (Gibco-BRL) were included in parallel lanes. The membranes were soaked in blocking solution (10 mM Tris-HCl, pH 7.2; 150 mM NaCl; 5% skim milk or 5% bovine serum albumin [BSA]; 0.02% NaN3) at 4°C overnight and then reacted with antibodies for 3 h at room temperature. The membranes were washed five times with washing buffer (10 mM Tris-HCl, pH 7.2; 150 mM NaCl; 0.3% Tween 20) and incubated for 1 h with alkaline phosphatase (AP)-conjugated secondary antibodies (KPL, Gaithersburg, Md.). Bound enzyme-labeled antibodies were detected by evaluating the color reaction of AP with nitroblue tetrazolium and BCIP (5-bromo-4-chloro-3-indolyphosphate) substrates (Sigma). The reactions were stopped by washing the membranes in distilled water.
Surface immunofluorescence assay (SIFA).
To detect the binding of gpK8.1A and ΔTMgpK8.1 to the cell surface, BJAB and 293 suspension cells or HFF and 293 cell monolayers in chamber slides were used (2, 60). A suspension of cells (107) was washed once with RPMI 1640, resuspended in 10 ml of ice-cold 0.1% paraformaldehyde in PBS (pH 7.4), and centrifuged at 125 × g for 10 min. The cells were washed twice, the concentration was adjusted to 106 cells per ml, the cells were centrifuged for 10 min at 125 × g and the supernatants were discarded. Dilutions of purified gpK8.1A (1 μg/ml) and ΔTMgpK8.1A (1 μg/ml) in 200 μl of RPMI 1640 with 10% FBS were added to the cell pellets, mixed, and incubated for 30 min at 37°C. These cells were washed five times with RPMI 1640 with 0.01% NaN3 and then incubated with prestandardized dilutions of gpK8.1A MAb or control antibodies for 30 min at 37°C. Cells were washed five times with RPMI 1640 with 0.01% NaN3 and incubated for 30 min at 37°C with prestandardized fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse or anti-rabbit IgG antibodies. Cells were washed and mounted on glass slides, and cell-bound gpK8.1A was examined under a fluorescence microscope. For binding with monolayers, cells were treated with 0.1% paraformaldehyde, washed five times with RPMI 1640, incubated with dilutions of purified proteins, and processed as described above.
Radiolabeled ΔTMgpK8.1A binding assays.
A protein binding assay was performed according to the method described previously (6), with minor modifications. Briefly, confluent monolayers of HFF, 293, HMVEC-d, CHO-K1, and CHO mutant derivative cells in 96- or 24-well plates were washed and blocked for 30 min at 4°C with PBS containing 1% FBS, 5 mM albumin, and 0.1 mM CaCl2. Cells were incubated with different concentrations of purified ΔTMgpK8.1A (7,666 cpm/μg of protein) or purified ORF 73 protein (14,672 cpm) in DMEM with 10% FBS and 0.01% NaN3 for 90 min at 4°C. After incubation, cells were washed five times with DMEM and lysed with 1% SDS and 1% Triton X-100 in distilled water, and the cell-bound ΔTMgpK8.1A radioactivity was counted. All experiments were done in triplicate and were repeated three times.
For homologous competition assays, confluent HFF cells were preincubated with different concentrations of nonlabeled ΔTMgpK8.1A for 15 min at 4°C and then incubated with 3.5 and 15 μg of labeled ΔTMgpK8.1A for the 96- and 24-well plates, respectively, for 90 min at 4°C. Cells were washed five times, lysed with 1% SDS and 1% Triton X-100 in distilled water, and counted. Each reaction was done in triplicate and repeated three times.
To test the ability of heparin to inhibit ΔTMgpK8.1A binding, a constant quantity of purified labeled ΔTMgpK8.1A (3.5 and 15 μg for the 96- and 24-well plates, respectively) was mixed with medium alone or medium with different concentrations of heparin and then incubated at 4°C for 90 min. These mixtures were then added to the target cells, followed by incubation at 4°C for 90 min, and then washed five times with DMEM and lysed with 1% SDS and 1% Triton X-100 in distilled water. The cell-bound ΔTMgpK8.1A counts per minute (cpm) in the presence or absence of heparin and the percentage of inhibition of binding were calculated. All reactions were done in triplicate and were repeated three times.
The specificity of HS binding was determined by incubating HFF cell monolayers with labeled ΔTMgpK8.1A (3.5 and 15 μg for the 96- and 24-well plates, respectively). At different time points, the cells were incubated with medium alone (controls) or with medium containing heparin (10 μg/ml). Cells were further incubated for 90 min at 4°C, washed five times, lysed with 1% SDS and 1% Triton X-100 in distilled water, and counted. The cell-associated ΔTMgpK8.1A cpm in the presence or absence of heparin and the percentage of inhibition of ΔTMgpK8.1A binding were calculated. All reactions were done in triplicate and were repeated three times.
Blocking HHV-8 binding by purified ΔTMgpK8.1A.
Radiolabeled HHV-8 binding assay was performed using HFF cells as per methods described previously (2). Briefly, HFF cells were incubated with increasing concentrations of purified unlabeled ΔTMgpK8.1A for 90 min at 4°C, followed by the addition of a fixed quantity of [3H]thymidine-labeled purified HHV-8 (2,684 cpm) (2). For a control, a fixed quantity of [3H]thymidine-labeled purified HHV-8 (2,684 cpm) was mixed with 10 μg of heparin per ml for 90 min at 4°C and then added to HFF cells. After incubation for 90 min at 4°C with the virus, cells were washed five times and lysed with 1% SDS and 1% Triton X-100, and the radioactivity was precipitated with trichloroacetic acid (TCA) and counted. The cell-associated virus cpm in the absence or presence of unlabeled ΔTMgpK8.1A or heparin and the percentage of inhibition of virus binding were calculated. All reactions were done in triplicate and repeated three times.
ΔTMgpK8.1A binding with heparin-agarose.
Purified ΔTMgpK8.1A (3.5 μg) was preincubated with 350 μg of various glycosaminoglycans (GAGs) such as heparin, HS, chondroitin sulfate A (CS-A), chondroitin sulfate B (CS-B), chondroitin sulfate C (CS-C), N-acetyl heparin, and de-N-sulfated heparin (Sigma). After being mixed for 1 h at 4°C, 100 μl of a 50% slurry of heparin-agarose beads (Sigma) equilibrated in radioimmunoprecipitation assay (RIPA) lysis buffer (0.05 M Tris hydrochloride, pH 7.5; 0.15 M NaCl; 1% sodium deoxycholate; 1% Triton X-100, 100 U of aprotinin per ml; 0.1 mM phenylmethylsulfonyl fluoride) (60) was added and further mixed for 2 h at 4°C. The heparin-agarose beads were washed five times in RIPA buffer. The bound material was eluted by boiling the beads in sample buffer with 2-ME, resolved by SDS–12%PAGE, Western blotted, and analyzed with anti-gpK8.1A MAb.
The specificity of ΔTMgpK8.1A binding to heparin-agarose beads was tested by preincubating purified ΔTMgpK8.1A with different concentrations of heparin for 1 h at 4°C and then incubating it with heparin-agarose beads for 2 h at 4°C. These mixtures were washed five times. The bound materials were eluted by boiling the beads in sample buffer and analyzed by Western blotting with anti-gpK8.1A MAb.
Virion envelope-associated gpK8.1A binding with heparin-agarose.
HHV-8 from TPA-induced BCBL-1 cells was purified by two cycles on a sucrose density gradient as per method described before (13). Purified virus was labeled with biotin according to the manufacturer's recommendations (Gibco-BRL), and the free biotin was removed by extensive dialysis against 0.5 M sodium carbonate buffer (pH 9.0) and then against PBS (pH 7.4). The cell-binding activity of biotin-labeled virus was tested by SIFA as described above, and bound virus was detected by use of gpK8.1A MAb or FITC-labeled streptavidin. To test the binding activity with heparin-agarose, biotin-labeled purified HHV-8 was lysed with RIPA buffer, sonicated, and centrifuged at 100,000 × g for 1 h at 4°C. The resulting soluble biotinylated envelope protein supernatant was mixed with 100 μl of 50% slurry of heparin-agarose or agarose beads in RIPA buffer and mixed for 2 h at 4°C. The beads were washed five times in RIPA buffer, boiled in sample buffer with 2-ME, resolved by SDS–10% PAGE, Western blotted, and analyzed with anti-gpK8.1A MAb or with AP-conjugated streptavidin (Dako, Carpinteria, Calif.).
Purification of HHV-8 full-length gpK8.1A.
Full-length gpK8.1A was purified using methods previously described (60). Briefly, TPA (Sigma, St. Louis, Mo.)-induced BCBL-1 cells were lysed on ice for 1 h with lysis buffer (10 mM Tris-HCl, pH 8.0; 140 mM NaCl; 0.025% NaN3; 2% Triton X-100; 1% sodium deoxycholate; 0.2 U of aprotinin per ml; 1 mM phenylmethylsulfonyl fluoride). Cell lysates were passed over a column of Sepharose 4B covalently coupled with gpK8.1A-specific MAb 4D6 at 4°C. The unbound proteins were removed by extensive washing with lysis buffer. The bound gpK8.1A was eluted with low-pH buffer (50 mM glycine-HCl [pH 2.5] in 150 mM NaCl and 0.1% NP-40) and immediately neutralized with a 1/10 volume of 1 M Tris-HCl (pH 8.0). The peak fractions were pooled, dialyzed against PBS (pH 7.0), and stored at −70°C.
RESULTS
HHV-8 envelope glycoprotein gpK8.1A binds to the target cells.
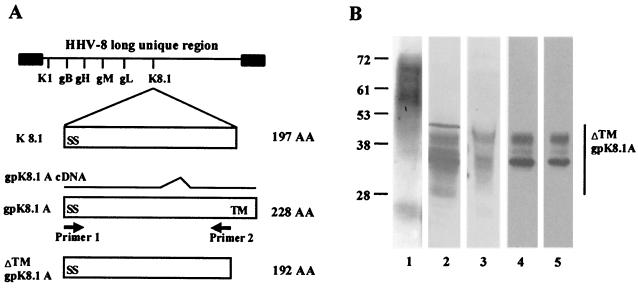
The HHV-8 K8.1 gene encodes two ORFs, gpK8.1A and gpK8.1B, which are derived from spliced mRNAs (10). The gpK8.1A ORF is 228 aa long with a signal sequence and a transmembrane domain, consisting of a 167-aa region identical to gpK8.1B and a unique 61-aa region (Fig. 1A). The amino-terminal 142-aa region of gpK8.1A is identical to the 197-aa genomic K8.1 ORF with the splicing event generating the gpK8.1A ORF transmembrane domain absent in the genomic K8.1 ORF (Fig. 1A) (10). HHV-8 gpK8.1A is a virion envelope-associated immunogenic glycoprotein containing both N- and O-linked sugars (60). MAbs against gpK8.1A recognized multiple proteins with molecular masses ranging from 34 to 72 kDa from BCBL-1 cells and 68- to 72-kDa proteins from the virion particles (60). These multiple proteins represent the precursor and glycosylated forms of gpK8.1A (60).
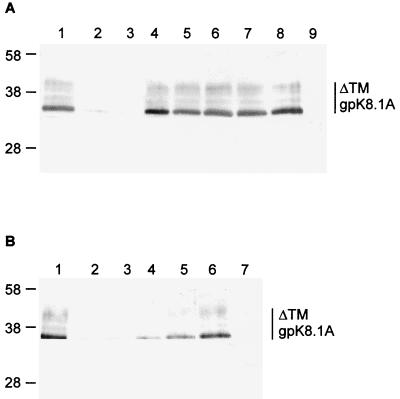
FIG. 1.
(A) Construction of ΔTMgpK8.1A without the transmembrane and carboxyl domains. The top line shows the schematic diagram of HHV-8 genome and the location of encoded glycoprotein ORFs. The genomic K8.1 ORF is 197 aa long with a signal sequence (SS) and without the transmembrane (TM) sequence. The 228-aa gpK8.1A ORF with signal and transmembrane sequences is derived from a spliced mRNA (10). The ΔTMgpK8.1A was constructed by using primers amplifying aa 1 to 192 with the signal sequence but lacking the transmembrane and the carboxyl domains. (B) Expression and purification of ΔTMgpK8.1A in the baculovirus expression system. Sf9 insect cells were infected with ΔTMgpK8.1A-baculovirus for 2 days and labeled with [35S]methionine for 20 h. His-tagged ΔTMgpK8.1A protein from the cell pellet was purified by use of a nickel column. Protein purity was analyzed by SDS–12% PAGE gels, Western blots with anti-gpK8.1 MAb, and autoradiography. Lane 1, full-length gpK8.1A affinity purified from HHV-8-infected BCBL-1 cells detected by Western blot reaction with anti-gpK8.1A MAb; lane 2, ΔTMgpK8.1A-expressing Sf9 insect cell lysate in Western blot reactions with anti-gpK8.1A MAb; lane 3, ΔTMgpK8.1A-expressing Sf9 cells culture supernatant in Western blot reactions with anti-gpK8.1A MAb; lane 4, [35S]methionine-labeled purified ΔTMgpK8.1A in Western blot reactions with anti-gpK8.1A MAb; lane 5, autoradiography of [35S]methionine-labeled purified ΔTMgpK8.1A. The numbers on the left indicate the molecular masses (in kilodaltons) of the standard protein markers run in parallel lanes. The glycosylated forms of ΔTMgpK8.1A are marked on the right.
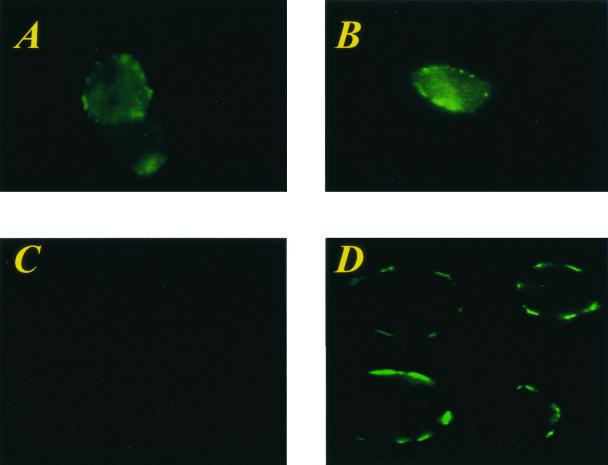
Because of the similarity of gpK8.1A to EBV gp350/gp220 involved in target cell recognition, we examined the ability of HHV-8 gpK8.1A to interact with the target cells. The gpK8.1A was affinity purified from TPA-induced BCBL-1 cells (Fig. 1B, lane 1) (60). Paraformaldehyde-fixed BJAB, 293, or HFF cells were incubated with purified gpK8.1A, washed, and reacted with anti-gpK8.1A MAbs or MAbs against HHV-8 ORF 59 (11) or normal mouse IgG. After incubation and washing, the bound antibody was detected by incubating with FITC-labeled anti-mouse IgG in SIFAs. Cells treated with 0.1% paraformaldehyde were used in the binding assay, since this treatment allows the binding of protein but prevents the entry into cells (25). In addition, binding assays can be performed at 37°C (25). Fluorescence signals representing the cell bound gpK8.1A were detected on the membranes of BJAB or 293 or HFF cells, and the results with BJAB and 293 cells are shown in Fig. 2A and B. No fluorescence signal was detected in cells incubated with anti-gpK8.1A MAbs only (Fig. 2C). Fluorescence signal was also not detected in cells incubated with ORF 59 MAbs or normal mouse IgG or rabbit antibodies against HHV-8 latency-associated ORF 73 protein (data not shown). Fluorescence signal was also not detected in cells incubated with His-tagged ORF 73 protein (data not shown). These results demonstrated the binding of gpK8.1A to the cell surface and suggested a role for gpK8.1A in the interaction between HHV-8 and the target cells.
FIG. 2.
HHV-8 gpK8.1A binds to the target cells. Binding of purified full-length gpK8.1A and ΔTMgpK8.1A to the target cells was detected by surface immunofluorescence assay. Paraformaldehyde-treated BJAB, 293, HFF, or HMVEC-d cells were incubated with medium alone (controls) or medium with purified proteins for 30 min at 37°C. After cells were washed, anti-gpK8.1A-specific MAb or anti-HHV-8 ORF 59 MAb (11) or rabbit anti-HHV-8 ORF 73 antibodies (34) were added, incubated for 30 min at 37°C, washed, and incubated for an additional 30 min at 37°C with FITC-conjugated goat anti-mouse or anti-rabbit IgG antibodies. Cells were washed, mounted, and examined under a fluorescence microscope. (A and B) BJAB and 293 cells, respectively, incubated with the full-length affinity-purified gpK8.1A and anti-gpK8.1A MAb. (C) BJAB cells incubated with anti-gpK8.1A MAb alone. (D) BJAB cells incubated with the purified His-tagged ΔTMgpK8.1A and anti-gpK8.1A MAb. Fluorescence signals detected on the surface of cells indicate the cell-bound gpK8.1A and ΔTMgpK8.1A.
Expression and purification of HHV-8 ΔTMgpK8.1A without transmembrane and cytoplasmic domains.
Since only about 20% of TPA-induced BCBL-1 cells expressed HHV-8 lytic-cycle proteins, the yield of purified gpK8.1A by affinity chromatography was insufficient for binding studies. Hence, a 576-bp ΔTMgpK8.1A gene region encoding aa 1 to 192 lacking the transmembrane and the carboxyl domains was amplified by PCR (Fig. 1A), cloned, and expressed in the baculovirus system. The ΔTMgpK8.1A-baculovirus-infected Sf9 cell pellets and the culture supernatant were analyzed in Western blot reactions with gpK8.1A-specific MAbs. The predicted molecular mass of unglycosylated ΔTMgpK8.1A is about 21 kDa. MAbs recognized proteins ranging from 29 to 42 kDa from the ΔTMgpK8.1A-baculovirus-infected Sf9 cell pellets and culture supernatant (Fig. 1B, lanes 2 and 3). These proteins represent the different glycosylated forms of ΔTMgpK8.1A. The molecular masses of baculovirus-expressed ΔTMgpK8.1A proteins (Fig. 1B, lanes 2 and 3) were smaller than the gpK8.1A from the BCBL-1 cells (Fig. 1B, lane 1). This could be due to the absence of 36 aa in ΔTMgpK8.1A, as well as to the differences in the efficiency of N and O glycosylation between insect and mammalian cells (60, 61). HHV-8 ORF 59 MAbs or normal mouse IgG did not react with ΔTMgpK8.1A protein in Western blot reactions (data not shown).
Sf9 cells infected with ΔTMgpK8.1A-baculovirus were labeled with [35S]methionine and radiolabeled ΔTMgpK8.1A from the cell lysate was purified by use of nickel columns. The purity of the protein was analyzed by silver staining of SDS-PAGE, Western blot reactions with anti-gpK8.1A MAb, and autoradiography. Fractions containing the purified protein were pooled, dialyzed, concentrated, and reanalyzed. Purified radiolabeled ΔTMgpK8.1A proteins of about 32 to 42 kDa were detected in the Western blot reactions and by autoradiography (Fig. 1B, lanes 4 and 5). Contaminating proteins were not detected. We used the ΔTMgpK8.1A protein purified from the Sf9 cell pellets in all subsequent assays, since only a limited quantity of ΔTMgpK8.1A was detected in the infected Sf9 cell culture supernatant. This could be due to the weak cleavage of gpK8.1A signal sequence in the insect cells. A similar observation was made when HSV gD was expressed with the native signal sequence (48).
HHV-8 ΔTMgpK8.1A binds to the target cells.
To determine whether ΔTMgpK8.1A binds to the target cells, unlabeled purified ΔTMgpK8.1A was allowed to bind the paraformaldehyde-treated BJAB, 293, HFF, or HMVEC-d cells, which were washed and tested with anti-gpK8.1A MAbs in SIFAs. Bright-ring-type fluorescence was observed only on cells incubated with ΔTMgpK8.1A, and the results with BJAB cells are shown in Fig. 2D. Binding was not detected when cells were incubated with purified His-tagged ORF 73 protein (data not shown). These data further confirm the interaction of gpK8.1A with the cell surface and show that the extracellular domains of gpK8.1A mediate this binding.
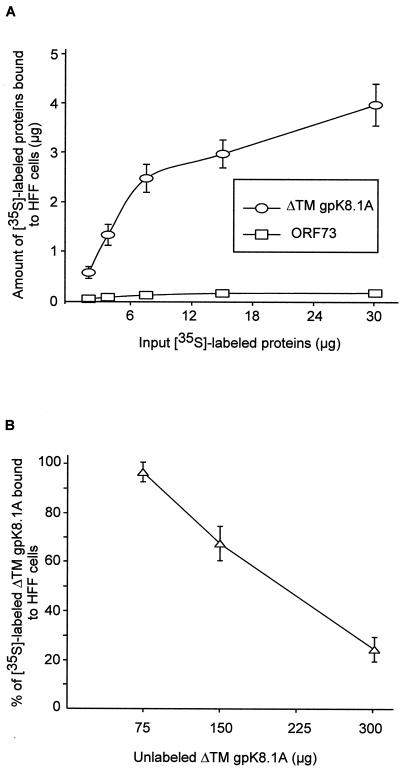
To quantitate the target cell bindings, purified [35S]methionine-labeled ΔTMgpK8.1A (7,666 cpm/μg of protein) was incubated with HFF, BJAB, 293, and HMVEC-d cells. Radiolabeled ΔTMgpK8.1A bound to all cells in a dose-dependent manner, and the results with HFF cells are shown in Fig. 3A. Similar results were observed when binding assays were performed with untreated cells at 4°C or with paraformaldehyde-treated cells at 37°C. The results with untreated cells at 4°C are presented here. Binding was not detected when the cells were incubated with [35S]methionine-labeled (14,672 cpm/μg of protein) purified His-tagged HHV-8 latency-associated ORF 73 protein (Fig. 3A).
FIG. 3.
(A) Binding of radiolabeled ΔTMgpK8.1A to HFF cells. Different concentrations of [35S]methionine-labeled purified ΔTMgpK8.1A (7,666 cpm/μg of protein) or ORF 73 (14,672 cpm/μg of protein) proteins were incubated for 90 min at 4°C with HFF cells in 96- or 24-well plates. After incubation, cells were washed five times and lysed with 1% SDS and 1% Triton X-100, and the cell-bound ΔTMgpK8.1A radioactivity was counted. Each reaction was done in triplicate and each point represents the average ± the standard deviation (SD) of three experiments. Similar results were seen with cells in 96- and 24-well plates, and the results with the 96-well plates are shown here. (B) Inhibition of labeled ΔTMK8.1A binding to cells by unlabeled ΔTMK8.1A protein. HFF cells were preincubated with the indicated concentrations of nonlabeled ΔTMgpK8.1A for 15 min and then incubated with 3.5 μg (for cells in the 96-well plate) or 15 μg (for cells in the 24-well plate) of 35S-labeled ΔTMgpK8.1A (7,666 cpm/μg of protein) for 90 min at 4°C. Cells were washed five times and lysed with 1% SDS and 1% Trition X-100, and the cell-bound ΔTMgpK8.1A radioactivity was counted. The cell-associated radiolabeled ΔTMgpK8.1A cpm in the presence or absence of unlabeled protein was calculated. In the absence of unlabeled ΔTMgpK8.1A protein, about 30% of the input labeled ΔTMgpK8.1A (1.1 and 4.5 μg for cells in the 96-well and 24-well plates, respectively) became associated with the cells. Each reaction was done in triplicate, and each point represents the average ± the SD of three experiments.
To determine the specificity of ΔTMgpK8.1A binding, homologous competition assays were done. HFF cells were preincubated for 15 min at 4°C with different concentrations of unlabeled ΔTMK8.1A and then incubated with a fixed concentration (3.5 and 15 μg for the 96- and 24-well plates, respectively) of purified radiolabeled ΔTMgpK8.1A (7,666 cpm/μg of protein). Similar results were observed with untreated cells at 4°C or with paraformaldehyde-treated cells at 37°C. The results with untreated cells in 96-well plates at 4°C are presented in Fig. 3B. In the absence of unlabeled ΔTMgpK8.1A protein, about 30% of the input labeled ΔTMgpK8.1A (1.1 and 4.5 μg for cells in the 96- and 24-well plates, respectively) became associated with the cells. The binding of labeled protein was inhibited in a dose-dependent manner by the preincubation with unlabeled ΔTMgpK8.1A, demonstrating the specificity of labeled ΔTMgpK8.1A interactions with the cell surface.
Purified ΔTMgpK8.1A blocks HHV-8 binding to the target cells.
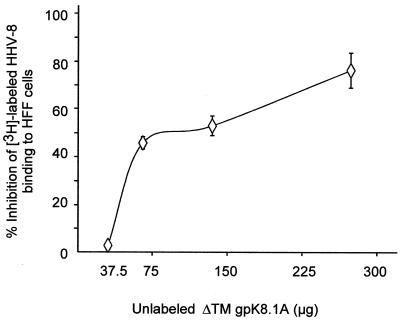
To determine if the interaction of gpK8.1A with the cell surfaces is biologically relevant, the ability of purified nonradiolabeled ΔTMgpK8.1A to complete with [3H]thymidine-labeled HHV-8 binding to HFF cells was examined. HFF cells were preincubated with increasing concentrations of purified unlabeled ΔTMK8.1A for 90 min at 4°C, followed by a constant quantity of [3H]thymidine-labeled purified HHV-8 (2,684 cpm), which is within the linear range of the dose-response curve (2). As a control for these experiments, a constant quantity of [3H]thymidine-labeled purified HHV-8 (2,684 cpm) was incubated with 10 μg of heparin per ml for 90 min at 4°C and then added to HFF cells. In the absence of heparin or unlabeled ΔTMgpK8.1A protein, approximately 21% of the input HHV-8 radioactivity became associated with the cells. As in to our earlier observation (2), 10 μg of heparin blocked approximately 90% of HHV-8 attachment to the cells (data not shown). HHV-8 adsorption was also blocked by the unlabeled purified ΔTMgpK8.1A in a dose-dependent fashion (Fig. 4). HHV-8 binding was diminished by approximately 70% compared to the untreated control (Fig. 4). Treatment of cells with identical concentrations of BSA or ORF 73 protein had no effect on virus binding, suggesting that the block in HHV-8 binding was probably to the engagement of a necessary HHV-8 cellular receptor by gpK8.1A and was not simply due to protein-protein interference. This suggested that gpK8.1A occupied cell surface molecule(s) that functions as an HHV-8 attachment receptor.
FIG. 4.
Nonradiolabeled ΔTMgpK8.1A blocks HHV-8 attachment. HFF cells were incubated with increasing concentrations of purified unlabeled ΔTMgpK8.1A for 90 min at 4°C, followed by the addition of a fixed quantity of [3H]thymidine-labeled purified HHV-8 (2,684 cpm) (2). For a control, a fixed quantity of [3H]thymidine-labeled purified HHV-8 (2,684 cpm) was mixed with 10 μg of heparin per ml for 90 min at 4°C and then added to HFF cells. After incubation for 90 min at 4°C with the virus, cells were washed five times and lysed with 1% SDS and 1% Triton X-100, and the radioactivity was precipitated with TCA and counted. The cell-associated virus cpm in the absence or presence of unlabeled ΔTMgpK8.1A and heparin and the percentage of inhibition of virus binding were calculated. In the absence of heparin or ΔTMgpK8.1A, approximately 21% of the input HHV-8 radioactivity (552 cpm) became associated with the cells. Approximately 90% of HHV-8 attachment to the cells was blocked by heparin. Each reaction was done in triplicate, and each point represents the average ± the SD of three experiments.
Heparin blocks HHV-8 ΔTMgpK8.1A binding to the target cells.
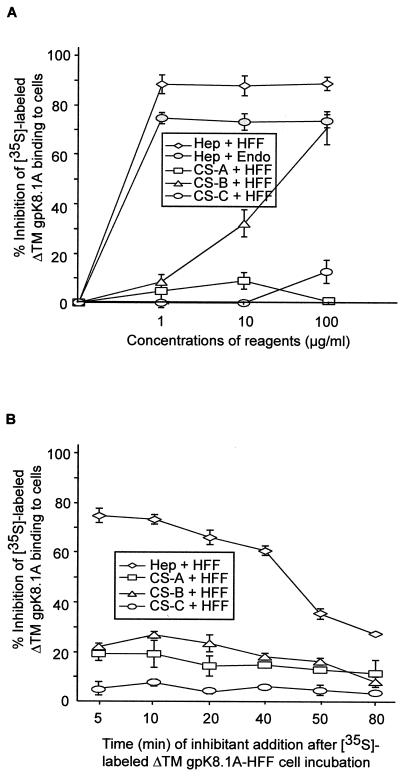
Heparin is closely related to HS, and inhibition of virus infectivity by heparin treatment has been considered as an evidence for alpha-, beta-, and gamma-2-herpesvirus interaction with cell surface HS molecules (21, 24, 30, 31, 33, 35, 37, 46–47, 49, 51, 57). Our recent studies showed that HHV-8 interaction with host cell surface involved HS and soluble heparin prevented HHV-8 infectivity (2). To determine whether heparin inhibits ΔTMgpK8.1A binding, a constant quantity of purified radiolabeled ΔTMgpK8.1A within the linear range of the dose-response curve (3.5 μg for cells in 96-well plates or 15 μg for cells in 24-well plates) (Fig. 3A) was mixed with medium alone or medium with different concentrations of heparin and incubated at 4°C for 90 min. These were then added to the paraformaldehyde-treated target cells and incubated at 37°C for 90 min or to the untreated target cells and incubated at 4°C for 90 min. After incubation, cells were washed five times and cell-associated ΔTMgpK8.1A cpm values were counted. Similar results were observed when binding assays were performed at 4 or at 37°C, and results with untreated cells 4°C are shown in Fig. 5A.
FIG. 5.
Inhibition of [35S]methionine-labeled purified ΔTMgpK8.1A binding to target cells by heparin. (A) A constant quantity of purified labeled ΔTMgpK8.1A (7,666 cpm/μg of protein) within the linear range of the dose-response curve (3.5 μg for cells in the 96-well plate or 15 μg for cells in the 24-well plate) (Fig. 3A) was mixed with medium alone or with different concentrations of heparin or CS-A, CS-B, or CS-C and then incubated for 90 min at 4°C. These mixtures were then incubated with HFF or adult HMVEC-d (Endo) for 90 min at 4°C and washed five times. Cells were lysed with 1% SDS–1% Triton X-100 and counted. The cell-associated ΔTMgpK8.1A cpm in the presence or absence of heparin and the percentage of inhibition of ΔTMgpK8.1A binding were calculated. In the absence of heparin, approximately 30% of the input ΔTMgpK8.1A radioactivity (1.1 and 4.5 μg for cells in the 96-well and 24-well plates, respectively) became associated with the cells. Each reaction was done in triplicate and each point represents the average ± the SD of three experiments. (B) Displacement of adsorbed ΔTMgpK8.1A from the HFF cell surface by heparin. HFF cell. monolayers in 96-well plates were incubated with a constant quantity (3.5 μg) of purified labeled ΔTMgpK8.1A (7,666 cpm/μg of protein). At the indicated time points, cells were incubated with medium (controls) or with medium containing 10 μg of heparin or CS-A, CS-B, or CS-C per ml. Cells were further incubated for a total of 90 min at 4°C, washed five times, and then counted. The cell-associated ΔTMgpK8.1A cpm in the presence or absence of heparin and the percentage of inhibition of ΔTMgpK8.1A binding were calculated. In the absence of heparin, approximately 30% of the input ΔTMgpK8.1A radioactivity (1.1 μg) became associated with the cells. Each reaction was done in triplicate, and each point represents the average ± the SD of three experiments.
In the absence of heparin, approximately 30% of the input ΔTMgpK8.1A radioactivity became associated with the cells. Soluble heparin significantly inhibited the binding of labeled ΔTMgpK8.1A to all cell lines tested in a dose-dependent manner. The results with HFF cells and HMVEC-d cells are shown in Fig. 5A. The percentage of inhibition plateaued at between 1 and 10 μg of heparin per ml for HFF and HMVEC cells (Fig. 5A) and for 293 cells (data not shown), and the maximum inhibition ranged from 83 to 95%. The specificity of heparin inhibition was shown by the absence of inhibition by CS-A and CS-C, even at a concentration of 100 μg/ml. CS-B also inhibited ΔTMgpK8.1A binding to the cell surface, with about 30 and 70% inhibition at concentrations of 10 and 100 μg/ml, respectively (Fig. 5A). However, these CS-B concentrations required to inhibit 50% of ΔTMgpK8.1A binding to the cell surface were almost 100 times higher than that of the required heparin concentration. The inhibition of ΔTMgpK8.1A binding to the target cells by heparin even at a low concentration suggested that ΔTMgpK8.1A interacts with the cell surface HS. The inability of heparin to completely prevent the protein binding suggests that gpK8.1A also binds to other host cell molecules.
Displacement of cell surface adsorbed HHV-8 ΔTMgpK8.1A by heparin.
To determine the specificity of HHV-8 ΔTMgpK8.1A interaction with cell surface HS and the inhibition by heparin, labeled ΔTMgpK8.1 was first allowed to adsorb to the HFF cells and at different times postadsorption, heparin or CS-A, -B, or -C were added to a final concentration of 10 μg/ml. Cells were further incubated for a total period of 90 min, and the cell-associated radioactivity was counted. Similar results were observed when binding assays were performed with paraformaldehyde-treated cells at 37°C or with untreated cells at 4°C. The results with untreated cells at 4°C are shown in Fig. 5. Pretreatment of HFF cells with heparin did not affect ΔTMgpK8.1 binding (data not shown). In the absence of heparin, about 30% of the input labeled ΔTMgpK8.1A (1.1 μg) became associated with the cells. In contrast, when heparin was added to the ΔTMgpK8.1A protein-cell mixture, it was capable of displacing already-adsorbed ΔTMgpK81A even when added 40 min after the protein addition to the cells (Fig. 5B). The partial reversal of binding by the addition of heparin after 50 min of protein-cell interaction (Fig. 5B) could be due to the onset of interactions between gpK8.1A and cellular receptors other than HS molecules. Reversal of ΔTMgpK8.1A binding to the cells by heparin demonstrated the specificity of HS interaction with HHV-8 gpK8.1A. Specificity was also shown by the absence of any significant inhibition by the same amount (10 μg/ml) of CS-A, -B, and -C (Fig. 5B).
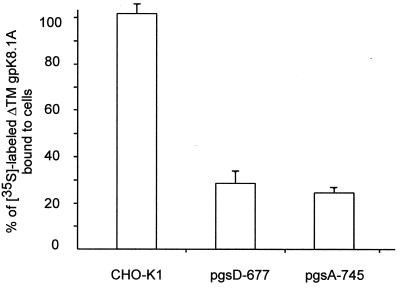
HHV-8 ΔTMgpK8.1A binds to the HS-expressing CHO-K1 cell line but not to cells lacking HS.
To verify the role of HS in the attachment of ΔTMgpK8.1A to the target cells, binding assays were done with wild-type CHO-K1 cell line expressing HS and its two mutant cell lines, pgsD-677 cells (deficient in HS but not in chondroitin sulfate) and pgsA-745 cells (deficient in both HS and chondroitin sulfate). Radiolabeled ΔTMgpK8.1A (3,310 cpm/μg of protein) bound readily to the wild-type CHO-K1 cells to the same extent as HFF cells. About 4 μg or 26% of the input labeled ΔTMgpK8.1A became associated with the CHO-K1 cells (Fig. 6). In contrast, ΔTMgpK8.1A binding to the mutant cells was significantly impaired, and about fivefold-less binding was detected with the pgsD-677 and pgsA-745 cells (Fig. 6). These results confirmed the interaction of HHV-8 ΔTMgpK8.1A with the cell surface HS. The low percentage of ΔTMgpK8.1A binding to the cells lacking HS further supported the notion that gpK8.1A also probably binds other host cell molecules.
FIG. 6.
Binding of radiolabeled ΔTMgpK8.1A to CHO-K1 cells. Confluent monolayers of wild-type CHO-K1 cells and of two CHO mutants, pgsD-677 (lacking HS but not chondroitin sulfate) and pgsA-745 (lacking both HS and chondroitin sulfate), in 24-well plates were incubated with 15 μg of [35S]methionine-labeled purified ΔTMgpK8.1A (3,310 cpm/μg of protein) for 90 min at 4°C. The cells were washed five times and lysed in 1% SDS–1% Triton X-100, and the cell-associated radioactivity was counted. About 4 μg or 26% of the input ΔTMgpK8.1A radioactivity bound to CHO-K1 cells. The results are expressed as the percentage of radioactivity bound to the wild-type CHO-K1 cells. Each reaction was done in triplicate, and each point represents the average ± the SD of three experiments.
HHV-8 ΔTMgpK8.1A specifically binds to heparin.
To verify the specificity of gpK8.1A binding to HS, the ability of ΔTMgpK8.1A to bind the heparin-agarose beads was tested. Purified ΔTMgpK8.1A, HHV-8 gL, or HHV-8 ORF 73 (2.5 μg) protein was incubated with heparin-agarose beads. After an extensive washing, the beads were boiled in sample buffer. The eluted proteins were detected by immunoblot using anti-gpK8.1A MAb, rabbit anti-gL antibodies, and rabbit anti-ORF 73 antibodies. Representative results are presented in Fig. 7. HHV-8 gL and ORF 73 proteins were not precipitated by heparin-agarose beads (data not shown). In contrast, heparin-agarose beads precipitated the various glycosylated forms of ΔTMgpK8.1A (Fig. 7A, lane 1). To determine the specificity of this reaction, various GAGs were tested to compete with the heparin-agarose binding activity of ΔTMgpK8.1A. Heparin-agarose binding activity of ΔTMgpK8.1A was competitively inhibited by preincubating the protein with 350 μg of heparin (Fig. 7A, lane 2) or 350 μg of HS (Fig. 7A, lane 3). In contrast, 350 μg of CS-A, -B, and -C, N-acetyl heparin, and de-N-sulfated heparin did not inhibit the ΔTMgpK8.1 interaction with the heparin-agarose beads (Fig. 7A, lanes 4 to 8). No reactivity was seen with agarose beads alone (Fig. 7A, lane 9), thus demonstrating the specificity of these reactions. These results confirmed the interaction of gpK8.1A with HS and heparin.
FIG. 7.
(A) HHV-8 ΔTMgpK8.1A binding to heparin-agarose beads. Purified ΔTMgpK8.1A (2.5 μg) was incubated with or without 350 μg of various GAGs for 1 h at 4°C and then with heparin-agarose beads for 2 h at 4°C. These mixtures were washed five times, and bound material was eluted by boiling in sample buffer, analyzed by SDS–12% PAGE gels, and tested with anti-gpK8.1A MAb in Western blot reactions. Lane 1, purified ΔTMgpK8.1A with heparin-agarose beads; lanes 2 to 8, purified ΔTMgpK8.1A preincubated with heparin (lane 2), HS (lane 3), CS-A (lane 4), CS-B (lane 5), CS-C (lane 6), N-acetyl heparin (lane 7), and de-N-sulfated heparin (lane 8) before the addition of heparin-agarose beads; lane 9, purified ΔTMgpK8.1A with agarose beads. The numbers on the left indicate the molecular masses (in kilodaltons) of the standard protein markers run in parallel lanes. The glycosylated forms of ΔTMgpK8.1A are marked on the right. (B) Dose-response results of heparin blocking HHV-8 ΔTMgpK8.1A binding to heparin-agarose beads. Purified ΔTMgpK8.1A (2.5 μg) was preincubated with different concentrations of heparin for 1 h at 4°C and then incubated with heparin-agarose beads for 2 h at 4°C. These mixtures were washed five times, and bound material was eluted by boiling the beads in sample buffer and then analyzed by SDS–12% PAGE gels and in Western blot reactions with anti-gpK8.1A MAb. Lane 1, purified ΔTMgpK8.1A with heparin-agarose beads; lanes 2 to 7, purified ΔTMgpK8.1A preincubated with 300 μg (lane 2), 150 μg (lane 3), 75 μg (lane 4), 38 μg (lane 5), or 19 μg (lane 6) of heparin before the addition of heparin-agarose beads; lane 7, purified ΔTMgpK8.1A with agarose beads. The numbers on the left indicate the molecular masses (in kilodaltons) of the standard protein markers run in parallel lanes. The glycosylated forms of ΔTMgpK8.1A are marked on the right.
The specificity of HHV-8 ΔTMgpK8.1A binding to heparin-agarose beads was also examined by preincubating 2.5 μg of purified ΔTMgpK8.1A with different concentrations of heparin for 1 h at 4°C and then incubating this with heparin-agarose beads for 2 h at 4°C. No reactivity was seen when agarose beads were incubated with purified ΔTMgpK8.1A (Fig. 7B, lane 7). Heparin-agarose beads precipitated the various glycosylated forms of ΔTMgpK8.1A (Fig. 7B, lane 1). This binding was completely inhibited by the preincubation with heparin at a concentration of 300 and 150 μg (Fig. 7B, lanes 2 and 3). Only moderate inhibition was seen with 75 and 38 μg of heparin (Fig. 7B, lanes 4 and 5), and no inhibition was seen with 19 μg of heparin (Fig. 7B, lane 6). These results further verified the interaction of gpK8.1A with HS and heparin.
Virion envelope-associated gpK8.1A binds heparin-agarose.
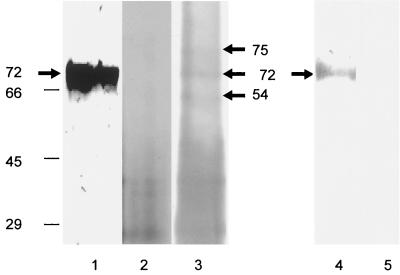
To determine whether virion envelope associated gpK8.1A binds heparin-agarose, density gradient-purified HHV-8 was labeled with biotin. The biotinylated virus bound to the target cells in the surface immunofluorescence assay (data not shown). Similar to our earlier findings (60), gpK8.1A MAbs recognized the 68- to 72-kDa protein in Western blot reactions with purified HHV-8 (Fig. 8, lane 1). Biotinylated purified virus was lysed with RIPA buffer, sonicated, and centrifuged at 100,000 × g. The resulting supernatant containing the soluble biotinylated envelope proteins was mixed with heparin-agarose or agarose beads for 2 h at 4°C and washed. The bound material was eluted by boiling in sample buffer, resolved by SDS-PAGE, Western blotted, and analyzed with AP-labeled streptavidin and substrate. Polypeptides of about 30 to 45 kDa precipitated both by agarose and by heparin-agarose were considered nonspecific bands (Fig. 8, lanes 2 and 3). Polypeptides of ca. 75, 72, and 54 kDa were specifically precipitated by heparin-agarose beads only (Fig. 8, lane 3). Preincubation of soluble biotinylated proteins with 350 μg of heparin or HS prevented the interaction of these specific proteins with heparin-agarose beads (data not shown). When the proteins precipitated by heparin-agarose beads were reacted with anti-gpK8.1A MAbs in the Western blots, only the 72-kDa protein was specifically recognized (Fig. 8, lane 4), and no reactivity was seen with agarose bead-precipitated proteins (data not shown). No reaction was seen when proteins precipitated by heparin-agarose beads were reacted with rabbit anti-gL IgG antibodies (Fig. 8, lane 5). The identity of the heparin-agarose interacting 75- and 54-kDa HHV-8 envelope glycoproteins is under investigation. These results demonstrated the ability of virion envelope-associated gpK8.1A to interact with heparin-agarose and thus HS.
FIG. 8.
Virion envelope-associated gpK8.1A binding with heparin-agarose. Biotin-labeled purified HHV-8 was lysed with RIPA buffer, sonicated, and centrifuged at 100,000 × g for 1 h at 4°C. The resulting supernatant containing soluble biotinylated envelope proteins was mixed with heparin-agarose or agarose beads, mixed for 2 h at 4°C, and washed five times in RIPA buffer. The bound material was eluted by boiling the beads in sample buffer with 2-ME, resolved by SDS–12% PAGE, Western blotted, and analyzed. Lane 1, purified virus solubilized by sample buffer in Western blot reactions with anti-gpK8.1A MAb; lane 2, biotinylated proteins eluted from the agarose beads reacted with AP-labeled streptavidin and substrate; lane 3, biotinylated proteins eluted from the heparin-agarose beads reacted with AP-labeled streptavidin and substrate; lane 4, biotinylated proteins eluted from the heparin-agarose beads in Western blot reactions with anti-gpK8.1A MAb; lane 5, biotinylated proteins eluted from the heparin-agarose beads in Western blot reactions with rabbit anti-HHV-8 gL IgG antibodies. The numbers on the left indicate the molecular masses (in kilodaltons) of the standard protein markers run in parallel lanes.
DISCUSSION
Proteoglycans are found abundantly in the extracellular matrices or cell surfaces of animal cells and mediate many fundamental cellular processes, including cell-to-cell and cell-to-matrix adhesion, motility, growth, and signaling (28). A proteoglycan is formed by the linkage of glycosaminoglycans such as HS or chondroitin sulfate to a protein core. HS is the initial binding target of many microorganisms, including parasites, bacteria, and viruses (23, 42, 54). Several alphaherpesviruses, such as HSV-1, HSV-2, PRV, and BHV-1 (21, 24, 30, 31, 33, 46, 47, 51, 59), betaherpesviruses, such as HCMV and HHV-7 (35, 37, 45, 49), and gamma-2-herpesviruses, such as BHV-4 (57), interact specifically with HS-like moieties. HS is also recognized by a wide spectrum of other viruses such as human immunodeficiency virus type 1 (38, 41), vaccinia virus (14), Sindbis virus (7), foot-and-mouth-disease virus (26), respiratory syncytial virus (19, 29), and adeno-associated virus (55).
Our recent studies show that the gamma-2-HHV-8, like some members of the alpha-, beta-, and gamma-2-herpesviruses, adsorbs to cells by binding to cell surface HS-like moieties (2). Studies here examined the role of HHV-8 envelope glycoprotein gpK8.1A in the interaction with target cells. Comparison with the human or animal herpesvirus sequences to date show that the gpK8.1A gene is unique for HHV-8. The location of the gpK8.1A gene in the genome clearly suggests an important role of gpK8.1A in the biology of HHV-8. The gpK8.1A gene is positionally colinear to the gamma-1-EBV gp350/gp220 gene (22), the gamma-2-MHV-68 gp150 gene (53), and the gamma-2-HVS ORF 51 gene (1). HHV-8 gpK8.1A shows several similarities with these proteins. Like EBV gp350/gp220 (56) and MHV-68 gp150 (53), HHV-8 gpK8.1A is a virion envelope- and infected cell membrane-associated glycoprotein (60). Antibodies against gp350/220 of EBV and gp150 MHV-68 neutralized the respective virus infectivities (53, 56). Binding of gpK8.1A to the target cells and the gpK8.1A blocking the radiolabeled HHV-8 binding shown here suggest that gpK8.1A plays an important role in the initial events of HHV-8 entry into susceptible cells. Our ongoing studies show that anti-gpK8.1A MAbs neutralize HHV-8 infectivity (data not shown). Inhibition of ΔTMgpK8.1A binding by heparin, binding of ΔTMgpK8.1A to the HS-expressing CHO-K1 cells, limited binding to the mutant derivatives of CHO cell lines lacking HS, specific binding of ΔTMgpK8.1A to HS but not to other GAGs, and the binding of virion gpK8.1A with heparin clearly demonstrate that gpK8.1A is involved in the interaction with HS. Even though heparin lowered the level of ΔTMgpK8.1A binding, the absence of complete inhibition suggests the interaction with other cell surface molecules. The low percentage of binding of ΔTMgpK8.1A to the CHO mutant cells lacking HS also reinforces this suggestion. Our results indicate that ΔTMgpK8.1 interaction with HS is the first important set of ligand-receptor interaction which may lead to the binding of one or more second receptor(s) essential for the subsequent viral entry process (23). The putative second receptor for gpK8.1A needs to be identified.
Inspection of the structure of heparin and/or HS and sequence analysis of the heparin-binding domain (HBD) of several proteins suggested that the negatively charged sulfate or carboxylate groups on heparin could interact via electrostatic interactions to positively charged cationic residues in a protein or peptide (28, 42, 54). HBDs are enriched with positively charged basic amino acids (lysine, arginine, and histidine). Two typical heparin motifs (XBBXBX and XBBBXXBX) have been identified, where “B” is a basic residue and “X” can be any other residue but is usually a hydrophobic residue (8). Analysis of amino acid sequence of gpK8.1A revealed two possible, although atypical heparin-binding motifs: gpK8.1A-H1 (150SRTTRIRV157, XBXXBXBX) and gpK8.1A-H2 (182TRGRDAHY189, XBXBXXBX). Whether these gpK8.1A putative HBDs play a role in the interaction with HS requires further investigation. It is also possible that several other weak and/or high-affinity HBDs may appear in HHV-8 gpK8.1A in its native quaternary structure, since the basic amino acids separated apart may lie juxtaposed, forming a typical HBD.
Among the eight HHVs, HS has been shown to mediate the attachment of HSV-1, HSV-2, HCMV, HHV-7, and HHV-8 (2, 21, 24, 30, 31, 33, 35, 37, 45–47, 49, 51, 59). In alphaherpesviruses, the glycoproteins gB and gC are known to bind cell surface HS (21, 24, 30, 31, 33, 51). The gC homologue of alphaherpesviruses is absent in the beta- and gammaherpesviruses, and the gBs of HCMV (betaherpesvirus) and BHV-4 (gammaherpesvirus) have been shown to mediate the HS binding of these viruses (35, 45, 57). Predictive analysis of HHV-8 sequence revealed the presence of putative HBD in HHV-8 gB. Ongoing studies show that HHV-8 envelope-associated gB also binds HS and the 75- and 54-kDa proteins precipitated by heparin-agarose from the biotinylated virus (Fig. 8, lane 2) represent the two cleaved-disulfide linked forms of HHV-8 gB (S. M. Akula et al. unpublished results). The presence of two or more heparin-binding glycoproteins within a single virus is not unexpected, since all well-studied human alpha- and betaherpesviruses contain at least two HS binding glycoproteins, e.g., gC and gB for HSV-1 and HSV-2, gB and gCII for HCMV, and gB and gp65 for HHV-7 (30, 31, 35, 37, 45, 49). The presence of two-HS binding proteins within the same virus indicates the importance of cell surface HS as receptors for viral attachment. HSV-1 gC and gB exhibit differences in their relative affinities for distinct cell surface HS proteoglycans (30). Whether HHV-8 gpK8.1A and gB also exhibit such differences needs to be studied.
ACKNOWLEDGMENTS
This study was supported in part by Public Health Service grant CA82056 to B.C.
We thank E. Stephens for critically reading the manuscript and for the use of the Nikon Magna firewire digital imaging system. We thank Clark Bloomer at the Biotechnology Center, University of Kansas Medical Center, Kansas City, for sequencing the DNA.
REFERENCES
- 1.Albrecht J C, Nicholas J, Biller D, Cameron K R, Biesinger B, Newman C, Wittmann S, Craxton M A, Coleman H, Fleckenstein B, Honness R W. Primary structure of the herpesvirus saimiri genome. J Virol. 1992;66:5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akula S M, Wang F-Z, Vierira J, Chandran B. Human herpesvirus 8 (HHV-8/KSHV) infection of target cells involves interaction with heparan sulfate. Virology. 2001;282:245–255. doi: 10.1006/viro.2000.0851. [DOI] [PubMed] [Google Scholar]
- 3.Ballestas M E, Chatis P A, Kaye K M. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science. 1999;284:641–644. doi: 10.1126/science.284.5414.641. [DOI] [PubMed] [Google Scholar]
- 4.Blasig C, Zietz C, Haar B, Neipel F, Esser S, Brockmeyer N H, Tschachler E, Colombini S, Ensoli B, Sturzl M. Monocytes in Kaposi's sarcoma lesions are productively infected by human herpesvirus 8. J Virol. 1997;71:7963–7968. doi: 10.1128/jvi.71.10.7963-7968.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boshoff C, Schulz T F, Kennedy M M, Graham A K, Fisher C, Thomas A, McGee J O, Weiss R A, O'Leary J J. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1:1274–1278. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- 6.Boyle K A, Compton T. Receptor-binding properties of a soluble form of human cytomegalovirus glycoprotein B. J Virol. 1998;72:1826–1833. doi: 10.1128/jvi.72.3.1826-1833.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrnes A P, Griffin D E. Binding of Sindbis virus to cell surface heparan sulfate. J Virol. 1998;72:7349–7356. doi: 10.1128/jvi.72.9.7349-7356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardin A D, Weintraub H J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- 9.Cesarman E, Moore P S, Rao P H, Inghirami G, Knowles D M, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- 10.Chandran B, Bloomer C, Chan S R, Zhu L, Goldstein E, Horvat R. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology. 1998;249:140–149. doi: 10.1006/viro.1998.9316. [DOI] [PubMed] [Google Scholar]
- 11.Chan S R, Bloomer C, Chandran B. Identification and characterization of Human herpesvirus-8 lytic cycle associated ORF 59 protein and the encoding cDNA by monoclonal antibody. Virology. 1998;240:118–128. doi: 10.1006/viro.1997.8911. [DOI] [PubMed] [Google Scholar]
- 12.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 13.Chatlynne L G, Lapps W, Handy M, Huang Y Q, Masood R, Hamilton A S, Said J W, Koeffler H P, Kaplan M H, Friedman-Kien A, Gill P S, Whitman J E, Ablashi D V. Detection and titration of human herpesvirus-8-specific antibodies in sera from blood donors, acquired immunodeficiency syndrome patients, and Kaposi's sarcoma patients using a whole virus enzyme-linked immunosorbent assay. Blood. 1998;92:53–58. [PubMed] [Google Scholar]
- 14.Chung C S, Hsiao J C, Chang Y S, Chang W. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J Virol. 1998;72:1577–1585. doi: 10.1128/jvi.72.2.1577-1585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decker L L, Shankar P, Khan G, Freeman R B, Dezube B J, Lieberman J, Thorley-Lawson D A. The Kaposi's sarcoma-associated herpesvirus (KSHV) is present as an intact latent genome in KS tissue but replicates in the peripheral blood mononuclear cells of KS patients. J Exp Med. 1996;184:283–288. doi: 10.1084/jem.184.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamond C, Brodie S J, Krieger J N, Huang M L, Koelle D M, Diem K, Muthui D, Corey L. Human herpesvirus 8 in the prostate glands of men with Kaposi's sarcoma. J Virol. 1998;72:6223–6227. doi: 10.1128/jvi.72.7.6223-6227.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, van Marck E, Salmon D, Gorin I, Escande J P, Weiss R A, Alitalo K, Boshoff C. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc Natl Acad Sci USA. 1999;96:4546–4551. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esko J D, Stewart T E, Taylor W H. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci USA. 1985;82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman S A, Audet S, Beeler J A. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J Virol. 2000;74:6442–6447. doi: 10.1128/jvi.74.14.6442-6447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flore O, Rafii S, Ely S, O'Leary J J, Hyjek E M, Cesarman E. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature. 1998;394:588–592. doi: 10.1038/29093. [DOI] [PubMed] [Google Scholar]
- 21.Flynn S J, Ryan P. The receptor-binding domain of pseudorabies virus glycoprotein gC is composed of multiple discrete units that are functionally redundant. J Virol. 1996;70:1355–1364. doi: 10.1128/jvi.70.3.1355-1364.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong M, Kieff E. Intracellular trafficking of two major Epstein-Barr virus glycoproteins, gp350/220 and gp110. J Virol. 1990;64:1507–1516. doi: 10.1128/jvi.64.4.1507-1516.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haywood A M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994;68:1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herold B C, Visalli R J, Susmarski N, Brandt C R, Spear P G. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J Gen Virol. 1994;75:1211–1222. doi: 10.1099/0022-1317-75-6-1211. [DOI] [PubMed] [Google Scholar]
- 25.Hutt-Fletcher L M, Balachandran N, LeBlane P A. Modification of Epstein-Barr virus replication by tunicamycin. J Virol. 1986;57:117–123. doi: 10.1128/jvi.57.1.117-123.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson T, Ellard F M, Ghazaleh R A, Brookes S M, Blakemore W E, Corteyn A H, Stuart D I, Newman J W, King A M. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J Virol. 1996;70:5282–5287. doi: 10.1128/jvi.70.8.5282-5287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kedes D H, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996;2:918–924. doi: 10.1038/nm0896-918. [DOI] [PubMed] [Google Scholar]
- 28.Kjellen L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 29.Krusat T, Streckert H J. Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch Virol. 1997;142:1247–1254. doi: 10.1007/s007050050156. [DOI] [PubMed] [Google Scholar]
- 30.Laquerre S, Argnani R, Anderson D B, Zucchini S, Manservigi R, Glorioso J C. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J Virol. 1998;72:6119–6130. doi: 10.1128/jvi.72.7.6119-6130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang X, Babiuk L A, Zamb T J. Mapping of heparin-binding structures on bovine herpesvirus 1 and pseudorabies virus gIII glycoproteins. Virology. 1993;194:233–243. doi: 10.1006/viro.1993.1254. [DOI] [PubMed] [Google Scholar]
- 32.Lidholt K, Weinke J L, Kiser C S, Lugemwa F N, Bame K J, Cheifetz S, Massague J, Lindahl U, Esko J D. A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc Natl Acad Sci USA. 1992;89:2267–2271. doi: 10.1073/pnas.89.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mettenleiter T C, Zsak L, Zuckermann F, Sugg N, Kern H, Ben-Porat T. Interaction of glycoprotein gIII with a cellular heparinlike substance mediates adsorption of pseudorabies virus. J Virol. 1990;64:278–286. doi: 10.1128/jvi.64.1.278-286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moses A V, Fish K N, Ruhl R, Smith P P, Strussenberg J G, Zhu L, Chandran B, Nelson J A. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J Virol. 1999;73:6892–6902. doi: 10.1128/jvi.73.8.6892-6902.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarro D, Paz P, Tugizov S, Topp K, La Vail J, Pereira L. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology. 1993;197:143–158. doi: 10.1006/viro.1993.1575. [DOI] [PubMed] [Google Scholar]
- 36.Neipel F, Albrecht J C, Fleckenstein B. Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J Virol. 1997;71:4187–4192. doi: 10.1128/jvi.71.6.4187-4192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neyts J, Snoeck R, Schols D, Balzarini J, Esko J D, Van Schepdael A, De Clercq E. Sulfated polymers inhibit the interaction of human cytomegalovirus with cell surface heparan sulfate. Virology. 1992;189:48–58. doi: 10.1016/0042-6822(92)90680-n. [DOI] [PubMed] [Google Scholar]
- 38.Patel M, Yanagishita M, Roderiquez G, Bou-Habib D C, Oravecz T, Hascall V C, Norcross M A. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res Hum Retrovir. 1993;9:167–174. doi: 10.1089/aid.1993.9.167. [DOI] [PubMed] [Google Scholar]
- 39.Renne R, Blackbourn D, Whitby D, Levy J, Ganem D. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J Virol. 1998;72:5182–5188. doi: 10.1128/jvi.72.6.5182-5188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 41.Roderiquez G, Oravecz T, Yanagishita M, Bou-Habib D C, Mostowski H, Norcross M A. Mediation of human immunodeficiency virus type 1 binding by interaction of cell surface heparan sulfate proteoglycans with the V3 region of envelope gp120-gp41. J Virol. 1995;69:2233–2239. doi: 10.1128/jvi.69.4.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rostand K S, Esko J D. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulz T F, Chang Y, Moor P S. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) In: McCance D J, editor. Human tumor viruses. Washington, D.C.: American Society for Microbiology; 1998. pp. 87–134. [Google Scholar]
- 45.Secchiero P, Sun D, De Vico A L, Crowley R W, Reitz M S, Jr, Zauli G, Lusso P, Gallo R C. Role of the extracellular domain of human herpesvirus 7 glycoprotein B in virus binding to cell surface heparan sulfate proteoglycans. J Virol. 1997;71:4571–4580. doi: 10.1128/jvi.71.6.4571-4580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shieh M T, Spear P G. Herpesvirus-induced cell fusion that is dependent on cell surface heparan sulfate or soluble heparin. J Virol. 1994;68:1224–1228. doi: 10.1128/jvi.68.2.1224-1228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shukla D, Liu J, Blaiklock P, Shworak N W, Bai X, Esko J D, Cohen G H, Eisenberg R J, Rosenberg R D, Spear P G. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 48.Sisk W P, Bradley J D, Leipold R J, Stoltzfus A M, Ponce de Leon M, Hilf M, Peng C, Cohen G H, Eisenberg R J. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J Virol. 1994;68:766–775. doi: 10.1128/jvi.68.2.766-775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skrincosky D, Hocknell P, Whetter L, Secchiero P, Chandran B, Dewhurst S. Identification and analysis of a novel heparin-binding glycoprotein encoded by human herpesvirus 7. J Virol. 2000;74:4530–4540. doi: 10.1128/jvi.74.10.4530-4540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith M S, Bloomer C, Horvat R, Goldstein E, Casparian J M, Chandran B. Detection of human herpesvirus 8 DNA in Kaposi's sarcoma lesions and peripheral blood of human immunodeficiency virus-positive patients and correlation with serologic measurements. J Infect Dis. 1997;176:84–93. doi: 10.1086/514043. [DOI] [PubMed] [Google Scholar]
- 51.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 52.Staskus K A, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney D, Anderson J, Ganem D, Haase A T. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart J P, Janjua N J, Pepper S D, Bennion G, Mackett M, Allen T, Nash A A, Arrand J R. Identification and characterization of murine gammaherpesvirus 68 gp150: a virion membrane glycoprotein. J Virol. 1996;70:3528–3535. doi: 10.1093/benz/9780199773787.article.b00034574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stringer S E, Gallagher J T. Heparan sulphate. Int J Biochem Cell Biol. 1997;29:709–714. doi: 10.1016/s1357-2725(96)00170-7. [DOI] [PubMed] [Google Scholar]
- 55.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thorley-Lawson D A, Geilinger K. Monoclonal antibodies against the major glycoprotein (gp350/220) of Epstein-Barr virus neutralize infectivity. Proc Natl Acad Sci USA. 1980;77:5307–5311. doi: 10.1073/pnas.77.9.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vanderplasschen A, Bublot M, Dubuisson J, Pastoret P P, Thiry E. Attachment of the gammaherpesvirus bovine herpesvirus 4 is mediated by the interaction of gp8 glycoprotein with heparinlike moieties on the cell surface. Virology. 1993;196:232–240. doi: 10.1006/viro.1993.1471. [DOI] [PubMed] [Google Scholar]
- 58.Vieira J, O'Hearn P, Kimball L, Chandran B, Corey L. Activation of Kaposi's sarcoma-associated herpesvirus (HHV8) lytic replication by human cytomegalovirus. J Virol. 2001;75:1378–1386. doi: 10.1128/JVI.75.3.1378-1386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.WuDunn D, Spear P G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu L, Puri V, Chandran B. Characterization of human herpesvirus-8 8-K8.1A/B glycoproteins by monoclonal antibodies. Virology. 1999;262:237–249. doi: 10.1006/viro.1999.9900. [DOI] [PubMed] [Google Scholar]
- 61.Zhu L, Wang R, Sweat A, Goldstein E, Horvat R, Chandran B. Comparison of human sera reactivities in immunoblots with recombinant human herpesvirus (HHV)-8 proteins associated with the latent (ORF73) and lytic (ORFs 65, K8.1A, and K8.1B) replicative cycles and in immunofluorescence assays with HHV-8 infected BCBL-1 cells. Virology. 1999;256:381–392. doi: 10.1006/viro.1999.9674. [DOI] [PubMed] [Google Scholar]