Abstract
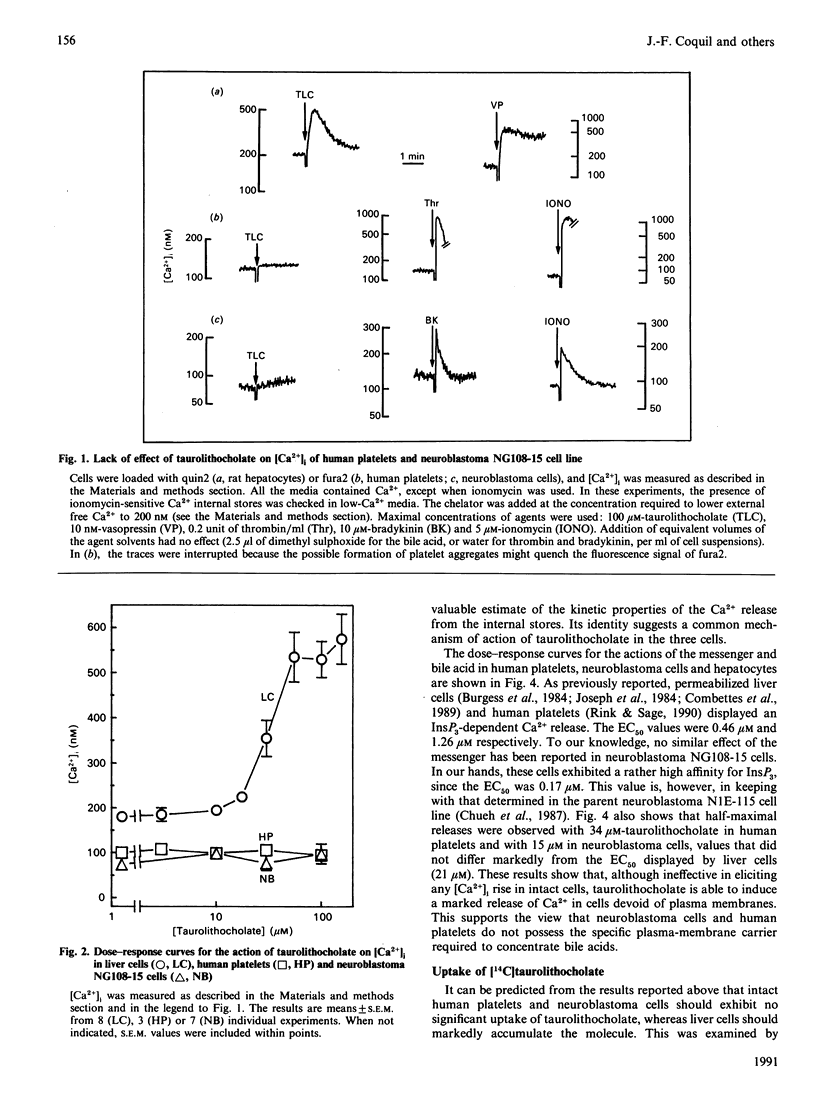
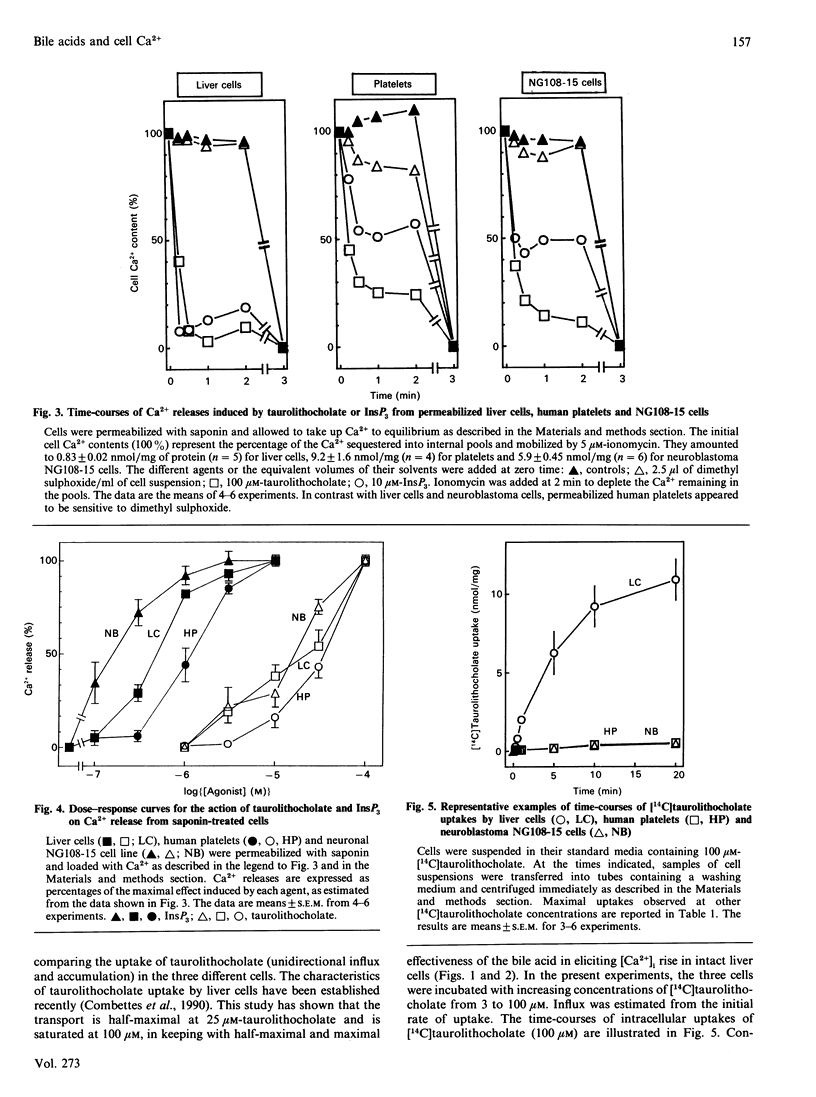
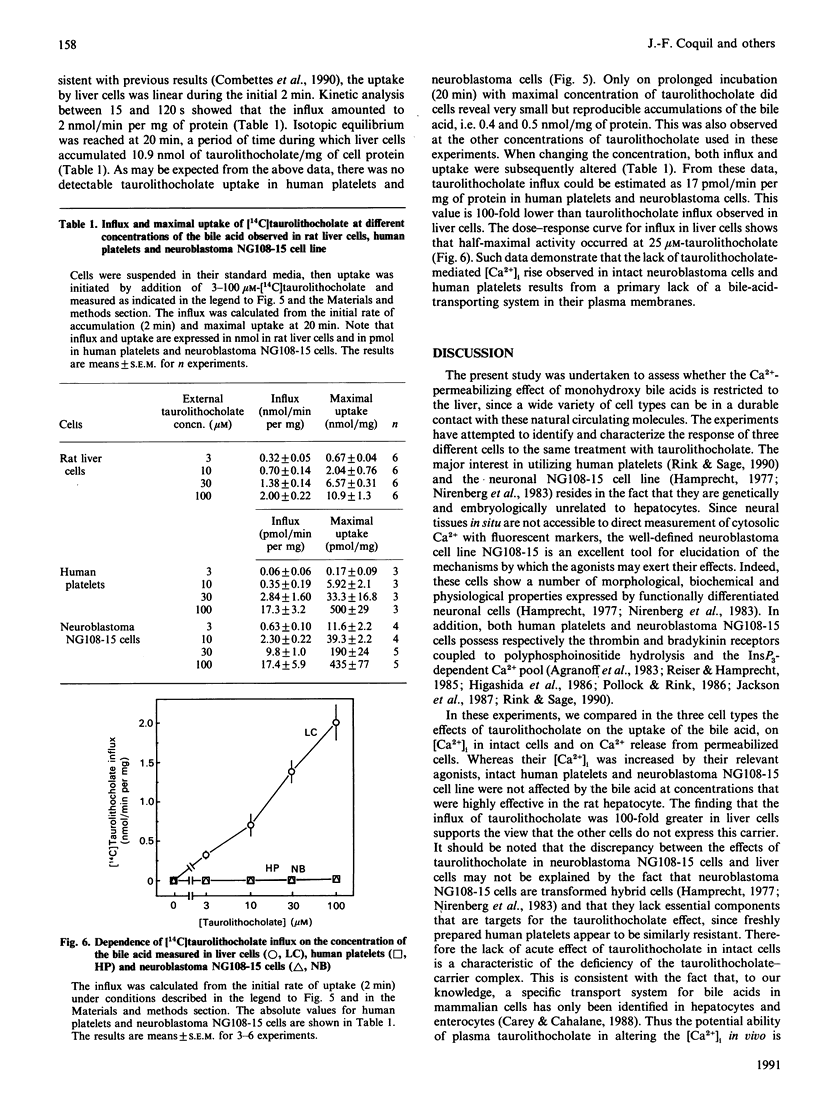
The monohydroxy bile acid taurolithocholate permeabilizes the endoplasmic reticulum to Ca2+ in rat liver cells. To assess whether this action on the endoplasmic reticulum was restricted to this tissue, the effects of bile acid were investigated in two cell types quite unrelated to rat hepatocyte, namely human platelets and neuronal NG108-15 cell line. The results showed that taurolithocholate (3-100 microM) had no effect on free cytosolic [Ca2+] in human platelets and NG108-15 cells. whereas it increased it from 180 to 520 nM in rat hepatocytes. In contrast, in cells permeabilized by saponin, taurolithocholate initiated a profound release of the stored Ca2+ from the internal Ca2+ pools in the three cell types. The bile acid released 90% of the Ca2+ pools, with rate constants of about 5 min-1 and half-maximal effects at 15-30 microM. The results also showed that, in contrast with liver cells, which displayed an influx of [14C]taurolithocholate of 2 nmol/min per mg, human platelets and the neuronal cell line appeared to be resistant to [14C]taurolithocholate uptake. The influx measured in these latter cells was about 100-fold lower than in rat liver cells. Taken together, these data suggest that human platelets and NG108-15 cells do not possess the transport system for concentrating monohydroxy bile acids into cells. However, they show that human platelets and neuronal NG108-15 possess, in common with liver cells, the intracellular system responsible for taurolithocholate-mediated Ca2+ release from internal stores.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W., Murthy P., Seguin E. B. Thrombin-induced phosphodiesteratic cleavage of phosphatidylinositol bisphosphate in human platelets. J Biol Chem. 1983 Feb 25;258(4):2076–2078. [PubMed] [Google Scholar]
- Ananthanarayanan M., von Dippe P., Levy D. Identification of the hepatocyte Na+-dependent bile acid transport protein using monoclonal antibodies. J Biol Chem. 1988 Jun 15;263(17):8338–8343. [PubMed] [Google Scholar]
- Anwer M. S., Engelking L. R., Nolan K., Sullivan D., Zimniak P., Lester R. Hepatotoxic bile acids increase cytosolic Ca++ activity of isolated rat hepatocytes. Hepatology. 1988 Jul-Aug;8(4):887–891. doi: 10.1002/hep.1840080430. [DOI] [PubMed] [Google Scholar]
- Anwer M. S., Little J. M., Oelberg D. G., Zimniak P., Lester R. Effect of bile acids on calcium efflux from isolated rat hepatocytes and perfused rat livers. Proc Soc Exp Biol Med. 1989 Jun;191(2):147–152. doi: 10.3181/00379727-191-42900. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Champeil P., Combettes L., Berthon B., Doucet E., Orlowski S., Claret M. Fast kinetics of calcium release induced by myo-inositol trisphosphate in permeabilized rat hepatocytes. J Biol Chem. 1989 Oct 25;264(30):17665–17673. [PubMed] [Google Scholar]
- Chueh S. H., Mullaney J. M., Ghosh T. K., Zachary A. L., Gill D. L. GTP- and inositol 1,4,5-trisphosphate-activated intracellular calcium movements in neuronal and smooth muscle cell lines. J Biol Chem. 1987 Oct 5;262(28):13857–13864. [PubMed] [Google Scholar]
- Coleman R. Biochemistry of bile secretion. Biochem J. 1987 Jun 1;244(2):249–261. doi: 10.1042/bj2440249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combettes L., Berthon B., Doucet E., Erlinger S., Claret M. Bile acids mobilise internal Ca2+ independently of external Ca2+ in rat hepatocytes. Eur J Biochem. 1990 Jul 5;190(3):619–623. doi: 10.1111/j.1432-1033.1990.tb15617.x. [DOI] [PubMed] [Google Scholar]
- Combettes L., Berthon B., Doucet E., Erlinger S., Claret M. Characteristics of bile acid-mediated Ca2+ release from permeabilized liver cells and liver microsomes. J Biol Chem. 1989 Jan 5;264(1):157–167. [PubMed] [Google Scholar]
- Combettes L., Dumont M., Berthon B., Erlinger S., Claret M. Effect of the bile acid taurolithocholate on cell calcium in saponin-treated rat hepatocytes. FEBS Lett. 1988 Jan 25;227(2):161–166. doi: 10.1016/0014-5793(88)80889-5. [DOI] [PubMed] [Google Scholar]
- Combettes L., Dumont M., Berthon B., Erlinger S., Claret M. Release of calcium from the endoplasmic reticulum by bile acids in rat liver cells. J Biol Chem. 1988 Feb 15;263(5):2299–2303. [PubMed] [Google Scholar]
- Frimmer M., Ziegler K. The transport of bile acids in liver cells. Biochim Biophys Acta. 1988 Feb 24;947(1):75–99. doi: 10.1016/0304-4157(88)90020-2. [DOI] [PubMed] [Google Scholar]
- Gill D. L., Chueh S. H. An intracellular (ATP + Mg2+)-dependent calcium pump within the N1E-115 neuronal cell line. J Biol Chem. 1985 Aug 5;260(16):9289–9297. [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Responses to adenosine diphosphate in human platelets loaded with the fluorescent calcium indicator quin2. J Physiol. 1985 Nov;368:131–146. doi: 10.1113/jphysiol.1985.sp015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamprecht B. Structural, electrophysiological, biochemical, and pharmacological properties of neuroblastoma-glioma cell hybrids in cell culture. Int Rev Cytol. 1977;49:99–170. doi: 10.1016/s0074-7696(08)61948-8. [DOI] [PubMed] [Google Scholar]
- Higashida H., Streaty R. A., Klee W., Nirenberg M. Bradykinin-activated transmembrane signals are coupled via No or Ni to production of inositol 1,4,5-trisphosphate, a second messenger in NG108-15 neuroblastoma-glioma hybrid cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):942–946. doi: 10.1073/pnas.83.4.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T. R., Hallam T. J., Downes C. P., Hanley M. R. Receptor coupled events in bradykinin action: rapid production of inositol phosphates and regulation of cytosolic free Ca2+ in a neural cell line. EMBO J. 1987 Jan;6(1):49–54. doi: 10.1002/j.1460-2075.1987.tb04717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Jourdon P., Berwald-Netter Y., Dubois J. M. Effects of dimethylsulfoxide on membrane currents of neuroblastoma x glioma hybrid cell. Biochim Biophys Acta. 1986 Apr 14;856(2):399–402. doi: 10.1016/0005-2736(86)90053-2. [DOI] [PubMed] [Google Scholar]
- Maenz D. D., Forsyth G. W. Calcium ionophore activity of intestinal secretory compounds. An in vitro porcine model for the effects of bile acids, hydroxy-fatty acids and dioctyl sulfosuccinate. Digestion. 1984;30(3):138–150. doi: 10.1159/000199098. [DOI] [PubMed] [Google Scholar]
- Matern S., Gerok W. Pathophysiology of the enterohepatic circulation of bile acids. Rev Physiol Biochem Pharmacol. 1979;85:125–204. doi: 10.1007/BFb0036117. [DOI] [PubMed] [Google Scholar]
- Mathis U., Karlaganis G., Preisig R. Monohydroxy bile salt sulfates: tauro-3 beta-hydroxy-5-cholenoate-3-sulfate induces intrahepatic cholestasis in rats. Gastroenterology. 1983 Sep;85(3):674–681. [PubMed] [Google Scholar]
- Meier P. J. The bile salt secretory polarity of hepatocytes. J Hepatol. 1989 Jul;9(1):124–129. doi: 10.1016/0168-8278(89)90085-8. [DOI] [PubMed] [Google Scholar]
- Meyer T., Wensel T., Stryer L. Kinetics of calcium channel opening by inositol 1,4,5-trisphosphate. Biochemistry. 1990 Jan 9;29(1):32–37. doi: 10.1021/bi00453a004. [DOI] [PubMed] [Google Scholar]
- Nirenberg M., Wilson S., Higashida H., Rotter A., Krueger K., Busis N., Ray R., Kenimer J. G., Adler M. Modulation of synapse formation by cyclic adenosine monophosphate. Science. 1983 Nov 18;222(4625):794–799. doi: 10.1126/science.6314503. [DOI] [PubMed] [Google Scholar]
- Oelberg D. G., Chari M. V., Little J. M., Adcock E. W., Lester R. Lithocholate glucuronide is a cholestatic agent. J Clin Invest. 1984 Jun;73(6):1507–1514. doi: 10.1172/JCI111356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelberg D. G., Dubinsky W. P., Sackman J. W., Wang L. B., Adcock E. W., Lester R. Bile salts induce calcium uptake in vitro by human erythrocytes. Hepatology. 1987 Mar-Apr;7(2):245–252. doi: 10.1002/hep.1840070207. [DOI] [PubMed] [Google Scholar]
- Oelberg D. G., Lester R. Cellular mechanisms of cholestasis. Annu Rev Med. 1986;37:297–317. doi: 10.1146/annurev.me.37.020186.001501. [DOI] [PubMed] [Google Scholar]
- Ogden D. C., Capiod T., Walker J. W., Trentham D. R. Kinetics of the conductance evoked by noradrenaline, inositol trisphosphate or Ca2+ in guinea-pig isolated hepatocytes. J Physiol. 1990 Mar;422:585–602. doi: 10.1113/jphysiol.1990.sp018002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock W. K., Rink T. J. Thrombin and ionomycin can raise platelet cytosolic Ca2+ to micromolar levels by discharge of internal Ca2+ stores: studies using fura-2. Biochem Biophys Res Commun. 1986 Aug 29;139(1):308–314. doi: 10.1016/s0006-291x(86)80114-0. [DOI] [PubMed] [Google Scholar]
- Reiser G., Hamprecht B. Bradykinin causes a transient rise of intracellular Ca2+-activity in cultured neural cells. Pflugers Arch. 1985 Oct;405(3):260–264. doi: 10.1007/BF00582570. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Sage S. O. Calcium signaling in human platelets. Annu Rev Physiol. 1990;52:431–449. doi: 10.1146/annurev.ph.52.030190.002243. [DOI] [PubMed] [Google Scholar]
- Schwenk M., Schwarz L. R., Greim H. Taurolithocholate inhibits taurocholate uptake by isolated hepatocytes at low concentrations. Naunyn Schmiedebergs Arch Pharmacol. 1977 Jun;298(2):175–179. doi: 10.1007/BF00508626. [DOI] [PubMed] [Google Scholar]
- Schölmerich J., Becher M. S., Schmidt K., Schubert R., Kremer B., Feldhaus S., Gerok W. Influence of hydroxylation and conjugation of bile salts on their membrane-damaging properties--studies on isolated hepatocytes and lipid membrane vesicles. Hepatology. 1984 Jul-Aug;4(4):661–666. doi: 10.1002/hep.1840040416. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Stark F., Nachmias V. T. Comparison of the effects of phorbol 12-myristate 13-acetate and prostaglandin E1 on calcium regulation in human platelets. Biochem J. 1988 Jan 15;249(2):487–493. doi: 10.1042/bj2490487. [DOI] [PMC free article] [PubMed] [Google Scholar]


