Abstract
Purpose
To evaluate the efficacy and safety of oral ibrexafungerp (HS-10366) versus placebo in Chinese patients with vulvovaginal candidiasis (VVC).
Methods
A double-blind, placebo-controlled, randomized, multicenter phase III study was conducted in symptomatic VVC patients. Patients received (2:1) twice-daily oral ibrexafungerp 300 mg or matching placebo for 1 day. The primary endpoint was clinical cure (vulvovaginal signs and symptoms [VSS] score = 0) at test-of-cure (TOC) on day 11 ± 3. The secondary endpoints included mycological eradication, overall response, and clinical improvement (VSS score ≤ 1) at TOC, and vulvovaginal symptom resolution at follow-up on day 25 ± 4.
Results
In total, 360 patients were included in the modified intention-to-treat set (defined as positive Candida cultured and receiving at least one study drug; 239 for ibrexafungerp, 121 for placebo). Compared with placebo, patients receiving ibrexafungerp had a significantly higher proportion of clinical cure (51.0% vs. 25.6%), mycological eradication (55.6% vs. 18.2%), overall response (33.9%, vs. 8.3%) at TOC and complete symptom resolution (74.5% vs. 39.7%, all P < 0.001) at follow-up. Subgroup analysis of clinical cure indicated that patients with C. albicans could benefit from ibrexafungerp over placebo. A similar benefit trend was also observed in those with non-albicans Candida by post-hoc analysis. Further analyses revealed similar efficacy of ibrexafungerp between patients with fluconazole non-susceptible C. albicans and fluconazole susceptible C. albicans regarding clinical cure and mycological eradication. Ibrexafungerp was generally well tolerated. Adverse events were primarily gastrointestinal and were mainly mild in severity.
Conclusions
As a first-in-class antifungal agent, ibrexafungerp demonstrated promising efficacy and favorable safety for VVC treatment in Chinese patients.
Chinadrugtrials.org.cn registry number
CTR20220918.
Supplementary Information
The online version contains supplementary material available at 10.1007/s15010-024-02233-w.
Keywords: Vulvovaginal candidiasis, Ibrexafungerp, Candida, Antifungal, Randomized clinical trial
Introduction
Vulvovaginal candidiasis (VVC) is a common fungal infection caused by Candida species and is a source of significant morbidity in women from all social classes. [1, 2]. About 75% of women will experience at least one episode of VVC, and 40 ~ 45% will experience multiple VVC episodes [1, 3]. The most common causative pathogen is C. albicans, and C. glabrata accounts for the most non-albicans Candida (NAC) [2]. Currently, azole antifungals are still preferable as the first-line therapy for VVC, including oral and topical formulations. Nevertheless, long-term use of azole antifungals and abuse of over-the-counter antifungals reduced fluconazole sensitivity among Candida species and increased drug resistance, especially for vaginal C. albicans isolates in China [4–7]. And there are limited options for VVC patients with azole non-susceptible Candida infection or with azole intolerance. Hence, new treatment approaches and agents possessing both broad-spectrum fungicidal activity and favorable safety profile, are urgently needed.
HS-10366 (generic name: ibrexafungerp) is a first-in-class, orally active, semisynthetic, triterpenoid derivative that blocks the synthesis of the fungal cell wall polymer β-(1,3)-d-glucan, which has broad-spectrum anti-Candida fungicidal activity, especially against echinocandin- and azole-resistant Candida species[8, 9]. The efficacy and safety of ibrexafungerp for treating VVC have been evaluated in two multicenter, global, randomized, double-blind, placebo-controlled phase III clinical trials in US and Bulgaria (VANISH 303, conducted in US, NCT03734991; VANISH 306, conducted in US and Bulgaria, NCT03987620). Both of trials demonstrated the superiority of ibrexafungerp over placebo in clinical cure, mycological eradication and overall success. Furthermore, ibrexafungerp was safe and generally well tolerated in VVC woman [10, 11].
Based on positive results of the VANISH 303/306 studies, ibrexafungerp received its first approval on 1 Jun 2021 in the US for the treatment of VVC in adult and post-menarchal pediatric females. The recommended dosage of ibrexafungerp is 300 mg twice daily for 1 day [12]. Furthermore, it was approved in the US for the prevention of recurrent vulvovaginal candidiasis (RVVC) in Nov 2022 based on another pivotal phase III clinical trial (CANDLE, NCT04029116) [12]. Ibrexafungerp is the only oral antifungal US FDA-approved treatment for VVC and reduction of RVVC.
Our study adopted a similar study design and the same dosage regimen as VANISH 303/306 study, which firstly intended to explore the efficacy and safety of ibrexafungerp in China’s VVC patients.
Methods
Study design and participants
This was a multicenter, randomized, double-blinded, placebo-controlled study. It aimed to assess the efficacy and safety of oral ibrexafungerp vs placebo among Chinese female patients with VVC. The pivotal study (Registry number: CTR20220918) was conducted at 31 tertiary hospitals in China (Appendix 1).
Female patients aged 18–64 (inclusive) were eligible if they were generally healthy and had a diagnosis of symptomatic VVC fulfilling the following criteria: (1) presenting at least two symptoms and/or signs that matched the vulvovaginal signs and symptoms (VSS) score (Supplementary Table 1) ≥ 2; (2) a microscopic result indicating positive Candida (showing hyphae/pseudohyphae/budding yeast); (3) vaginal pH ≤ 4.5 (considered as normal pH). Patients were excluded if they were pregnant, or concomitant with uncontrolled diabetes (HbA1c > 9%), other vaginal infections, or immunosuppression. A full version of the eligibility criteria is displayed in Appendix 2.
Eligible patients were randomly assigned in a 2:1 ratio to receive ibrexafungerp 300 mg (two 150-mg tablets) or matching placebo BID for 1 day. At randomization, patients were stratified by the diagnosis of diabetes mellitus (yes/no). A centralized, interactive response system was adopted for the randomization procedure. All patients, site staff and sponsor personnel were blinded to treatment assignment, except for a sponsor representative responsible for drug distribution. Placebo tablets were made indistinguishable from ibrexafungerp tablets.
The study was conducted under the guiding principles of the Declaration of Helsinki, Good Clinical Practice, and the current International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Guidelines. Written informed consent was obtained from all participants before the initiation of any study procedure.
Study assessments
The study consisted of screening (day − 2 ~ − 1), baseline (day 1), test-of-cure (TOC, day 11 ± 3), and follow-up (FU, day 25 ± 4) visits. Screening and baseline visits may occur on the same day. Rating of each vulvovaginal symptom (itching, pain) of VSS score was recorded by the subject in a diary from day 1 to the TOC visit, and the procedure was done under the supervision of the investigator at screening, TOC and FU visits. Each vulvovaginal sign (congestion/edema, scratches/rhagades/erosions, secretion volume) of the VSS score was rated by the investigator based on physical examinations at screening and TOC visits, and at FU visit only when the patient was symptomatic. VSS score was calculated at each visit.
Mycological evaluation for vulvovaginal samples included fungal cultures, vaginal pH test, and microscopic examination. Vaginal pH test and microscopic examination should be performed at the local labs of study centers at screening, and at TOC and FU visits when the patient was symptomatic. Fungal cultures and susceptibility tests should be performed at central laboratories at screening and TOC visits, and at FU visit if symptomatic. Susceptibility tests were performed under Clinical and Laboratory Standards Institute (CLSI) M59 and M60 guidelines [13, 14].
The patient could return to study centers for rescue therapy if she experienced persistence, worsening, or recurrence of symptoms ideally 48–72 h after first dose of the study drug. If rescue antifungal therapy was administered before or at TOC visit, the patient would be considered a failure for the efficacy endpoints evaluated at TOC visit due to lack of efficacy. Clinical and mycological assessments should be conducted at the discretion of investigators before the prescription of rescue antifungal agents (e.g., fluconazole).
Endpoints
The primary efficacy endpoint was the proportion of patients who reached clinical cure (VSS score = 0) at TOC visit. Main secondary efficacy endpoints included the proportion of patients with mycological eradication, overall response (achieving both clinical cure and mycological eradication), and clinical improvement at TOC visit, as well as vulvovaginal symptom resolution at FU visit. The definition of efficacy outcomes above was summarized in Supplementary Table 2. Safety endpoints focused on the incidence of treatment-emergent adverse events (TEAE), treatment-related treatment-emergent adverse events (TRAE), serious adverse events (SAE), and TEAE leading to study discontinuation.
Given an increasing emergence of NAC species and fluconazole-resistant C. albicans, post-hoc analyses for efficacy endpoints were conducted in patients infected by fluconazole susceptible and non-susceptible C. albicans and other Candida species.
Statistical analyses
All analyses were performed using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA). A sample size of 258 was calculated to provide about 90% power to detect a difference between Ibrexafungerp and placebo based on Pearson’s Chi-squared test with a type I error of 5%, an assumed clinical cure rate of 56.9% for ibrexafungerp and 35.7% for placebo, and a 2:1 randomization ratio. With an estimated 30% of patients without mycological culture-confirmed infection at baseline, a sample size of 369 (Ibrexafungerp, n = 246; placebo, n = 123) was planned to be randomized. Diagnosis of diabetes mellitus was used as a stratification factor during randomization.
Categorical variables were summarized by counts and percentages. Means (± SD) were used to descriptively summarize continuous variables. A 2-sided alpha of 0.05 was used for all hypothesis tests. Safety analyses were conducted for the safety set, which included randomized patients who received ≥ 1 dose of study drug. Cochran–Mantel–Haenszel test adjusted for diagnosis of diabetes mellitus was used for efficacy analyses primarily based on the modified intent-to-treat (mITT) population, which consisted of patients in the safety population who had a positive culture for Candida species at baseline. Patients who received rescue antifungal treatment on or before a specific visit and patients who were missing categorical response data at specific visit were considered to be non-responders. Three sensitivity analyses were also performed: Strategy 1 performed multiple imputation (MI) for 100 times in patients lacking clinical cure data at TOC visit due to COVID-19; Strategy 2 performed Copy Reference MI for 100 times in patients lacking clinical cure data at TOC visit; Strategy 3 only included patients with collected clinical cure response data or those who received rescue antifungal treatment before or at TOC visit (considered as failure). Details of the three strategies are described in Supplementary Table 3. Subgroup analyses were performed for BMI category at screening (< 28, ≥ 28 kg/m2), diagnosis of diabetes mellitus, severity of infection at baseline (mild to moderate, severe), and Candida species at screening.
Role of the funding source
The study was sponsored by Jiangsu Hansoh Pharmaceutical Group Co. Ltd (China). The sponsor was involved in study design, study monitoring, data collection, data analysis and interpretation, reporting of the study, and authorization of study results for publication.
Results
Patients
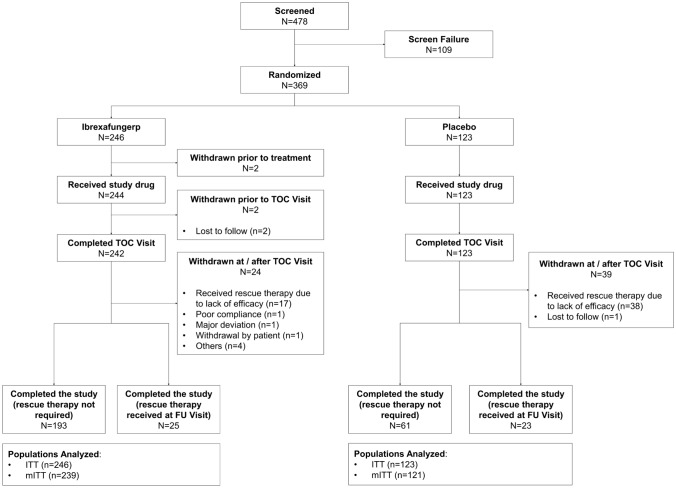
Patients were enrolled between August 2022 and February 2023 at 31 study sites in China. A total of 369 patients were randomized and assigned to either the ibrexafungerp group (n = 246) or placebo group (n = 123), among which 360 patients comprised the mITT set for primary efficacy analysis (239 for ibrexafungerp and 121 for placebo) (Fig. 1).
Fig. 1.
Patient disposition. TOC test-of-cure, FU follow-up, ITT intention-to-treat, mITT modified intention-to-treat
Demographic characteristics such as age, body mass index (BMI), proportion of menopause as well as diabetic patients between the treatment groups were comparable (Table 2). Most patients in both groups were of childbearing age, had a normal BMI range, and non-diabetic. There was also a similar pattern of VVC infection regarding severity and mean & median VSS score between the two groups at baseline. Over 80% of patients suffered mild-to-moderate VVC (VSS score < 7) and the rest suffered severe VVC (VSS score ≥ 7). The most common species cultivated at baseline was C. albicans (67.4% for ibrexafungerp and 62.0% for placebo), followed by C. glabrata and C. krusei (Table 2), which was consistent with the recent epidemiology of VVC in Chinese patients [6]. At screening, 20.0% (48/239) and 15.7% (19/121) patients were infected with fluconazole non-susceptible C. albicans in the ibrexafungerp and placebo groups, respectively (Table 1).
Table 2.
Summary of primary and secondary endpoints (mITT Set)
| Study endpoints | Ibrexafungerp, n/N (%) | Placebo, n/N (%) | Rate difference (%) (95%CI) | P value |
|---|---|---|---|---|
| Primary endpoint | ||||
| Clinical cure at TOC | 51.0 (122/239) | 25.6 (31/121) | 25.3 (15.31, 35.21) | < 0.001 |
| Secondary endpoints | ||||
| Mycological eradication at TOC | 55.6 (133/239) | 18.2 (22/121) | 37.5 (28.18, 46.88) | < 0.001 |
| Overall response at TOC | 33.9 (81/239) | 8.3 (10/121) | 25.6 (17.84, 33.38) | < 0.001 |
| Clinical improvement at TOC | 82.0 (196/239) | 48.8 (59/121) | 33.2 (23.03, 43.30) | < 0.001 |
| Complete symptom resolution at FU | 74.5 (178/239) | 39.7 (48/121) | 34.8 (24.45, 45.11) | < 0.001 |
mITT modified intention-to-treat, CI confidence interval, TOC test-of-cure, FU follow-up
Table 1.
Baseline characteristics (mITT set)
| Ibrexafungerp (N = 239) | Placebo (N = 121) | |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 32.4 (7.72) | 33.5 (8.54) |
| Median | 32.0 | 34.0 |
| Min, max | 18, 53 | 18, 58 |
| Ethnic group, n (%) | ||
| Han Chinese | 208 (87.0) | 105 (86.8) |
| Others | 31 (13.0) | 16 (13.2) |
| BMI (kg/m2) | ||
| Mean (SD) | 21.8 (3.14) | 22.5 (3.65) |
| Median | 21.4 | 21.6 |
| Min, max | 15.8, 34.5 | 17.0, 34.2 |
| BMI category, n (%) | ||
| < 28 kg/m2 | 228 (95.4) | 110 (90.9) |
| ≥ 28 kg/m2 | 11 (4.6) | 11 (9.1) |
| Menopause, n (%) | ||
| Yes | 3 (1.3) | 7 (5.8) |
| No | 236 (98.7) | 114 (94.2) |
| Diabetes mellitus, n (%) | ||
| Yes | 7 (2.9) | 3 (2.5) |
| No | 232 (97.1) | 118 (97.5) |
| Baseline VSS score | ||
| Mean (SD) | 4.9 (2.14) | 4.6 (1.77) |
| Median | 5.0 | 5.0 |
| Min, max | 2, 15 | 2, 11 |
| Severity based on VSS Score, n (%) | ||
| Mild to moderate (< 7) | 195 (81.6) | 106 (87.6) |
| Severe (≥ 7) | 44 (18.4) | 15 (12.4) |
| Cultured Candida spp. at screening, n (%)a | ||
| C. albicans | 161 (67.4) | 75 (62.0) |
| FLU susceptible C. albicans | 113 (47.3) | 56 (46.3) |
| FLU non-susceptible C. albicansb | 48 (20.1) | 19 (15.7) |
| C. glabrata | 60 (25.1) | 38 (31.4) |
| C. krusei | 9 (3.8) | 4 (3.3) |
| Other speciesb | 9 (3.8) | 5 (4.1) |
FLU fluconazole, mITT modified intent-to-treat, SD standard deviation, BMI body mass index, VVC vulvovaginal candidiasis, VSS vulvovaginal signs and symptoms
aPatients may have more than 1 Candida species at screening and would be counted once at each species level
bFluconazole non-susceptible C. albicans included both susceptible-dose dependent strains and resistant strains per Clinical and Laboratory Standards Institute M60 guideline
cOther species included C. parapsilosis, C. tropicalis, C. metapsilosis and C. inconspicua
Efficacy endpoints
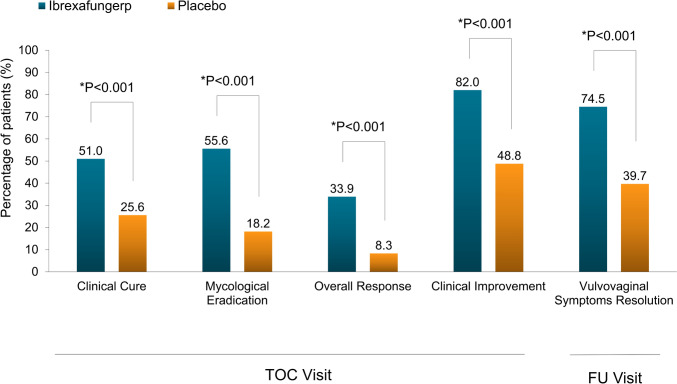
As primary endpoint, the clinical cure rate at TOC visit was significantly higher with ibrexafungerp (51.0%, 122/239) than placebo (25.6%, 31/121; difference [95% CI]: 25.3% [15.31, 35.21]; P < 0.001) (Table 2; Fig. 2). The result of clinical cure was further supported by sensitivity analysis (Supplementary Table 3).
Fig. 2.
Results of primary and secondary efficacy endpoints in the mITT set. mITT modified intention-to-treat
Ibrexafungerp demonstrated statistical superiority over placebo in all secondary endpoints (Table 2; Fig. 2). At TOC visit, mycological eradication (negative culture for Candida species) rate was significantly higher in the ibrexafungerp group than that of in the placebo group (55.6% [133/239] vs. 18.2% [22/121]; difference [95% CI] 38.8% [29.46, 48.21]; P < 0.001). Ibrexafungerp also demonstrated superiority over placebo in the percentage of patients achieving overall response (clinical cure and mycological eradication) at TOC visit (33.9% [81/239] vs. 8.3% [10/121]; difference [95% CI] 25.6% [17.84, 33.38]; P < 0.001), as well as patients with clinical improvement (VSS score ≤ 1) at TOC visit (82.0% [196/239] vs. 48.8% [59/121]; difference [95% CI] 33.2% [23.03, 43.30]; P < 0.001).
At FU visit, a larger proportion of patients in the ibrexafungerp group achieved vulvovaginal symptom resolution (74.5% [178/239] vs. 39.7% [48/121], P < 0.001) (Table 2; Fig. 2). Patients who missed FU visits were considered as failures. A majority of patients achieving clinical cure at TOC visit were also vulvovaginal asymptomatic at FU visit (Supplementary Table 4).
Subgroup analyses of clinical cure were performed as below. The superiority of ibrexafungerp over placebo was shown in both severe VVC patients (45.5% vs. 13.3%, P = 0.026) and mild-to-moderate VVC patients (52.3% vs. 27.4%, P < 0.001) (Table 3). For clinical cure, the efficacy of ibrexafungerp was also observed in non-obese (BMI < 28 kg/m2) and non-diabetic subgroups, while the efficacy could not be determined in obese and diabetic subgroups due to a limited sample size (22 obese patients and 10 diabetic patients were enrolled).
Table 3.
Subgroup analyses for primary endpoint (mITT Set)
| Subgroup | Clinical cure, n/N (%) | Rate difference (%), 95% CI | P value | |
|---|---|---|---|---|
| Ibrexafungerp | Placebo | |||
| Body mass index | ||||
| < 28 kg/m2 | 120/228 (52.6) | 29/110 (26.4) | 26.3 (15.01, 37.09) | < 0.001 |
| ≥ 28 kg/m2 | 2/11 (18.2) | 2/11 (18.2) | 0.0 (− 43.56, 43.56) | 1.000 |
| Diagnosis of diabetes | ||||
| Yes | 6/7 (85.7) | 2/3 (66.7) | 19.0 (− 47.08, 78.59) | 0.490 |
| No | 116/232 (50.0) | 29/118 (24.6) | 25.4 (14.49, 36.06) | < 0.001 |
| Severity of VVC | ||||
| Mild-to-moderate (VSS score < 7) | 102/195 (52.3) | 29/106 (27.4) | 24.9 (13.27, 36.15) | < 0.001 |
| Severe (VSS score ≥ 7) | 20/44 (45.5) | 2/15 (13.3) | 32.1 (2.14, 58.12) | 0.026 |
| Candida species at screeninga | ||||
| C. albicans | 88/161 (54.7) | 15/75 (20.0) | 34.7 (21.31, 47.17) | < 0.001 |
| C. glabrata | 26/60 (43.3) | 13/38 (34.2) | 9.1 (− 11.21, 28.95) | 0.369 |
| C. krusei | 3/9 (33.3) | 2/4 (50.0) | − 16.7 (− 70.15, 43.30) | 0.569 |
CI confidence interval, VVC vulvovaginal candidiasis, VSS vulvovaginal signs and symptoms
aSubgroup analyses would not be performed for subgroups containing fewer than ten patients
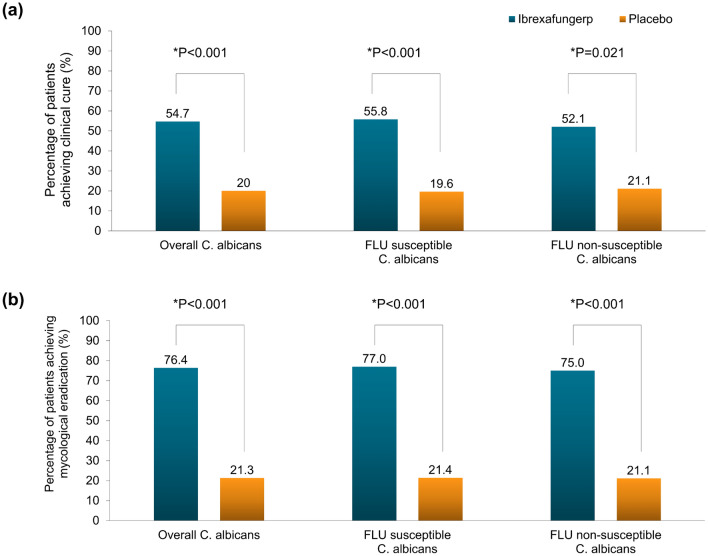
In the subgroup of those with C. albicans, a statistically significant result of clinical cure was found (54.7% [88/161] vs. 20.0% [15/75], P < 0.001), generally in line with results in the overall mITT set (Table 3). For mycological eradication, the rate difference was 55.1% (76.4% vs. 25.3%, P < 0.001) in those with C. albicans (Supplementary Table 5).
Due to an increasing proportion of fluconazole-resistant Candida in VVC infection in China, post-hoc analyses were explored in patients infected with fluconazole susceptible or non-susceptible C. albicans (per minimal inhibitory concentration [MIC]), and in patients infected with NAC. In the fluconazole non-susceptible C. albicans subgroups, ibrexafungerp showed superior efficacy over placebo regarding both clinical cure (52.1% vs. 21.1%, P = 0.021) and mycological eradication (75.0% vs. 21.1%, P < 0.001) (Supplementary Table 5; Supplementary Table 6). In the ibrexafungerp group, there were similar efficacy results between fluconazole non-susceptible C. albicans subgroup and overall C. albicans subgroup (Fig. 3A, B). There was also a trend that patients infected with NAC had a higher clinical cure rate in the ibrexafungerp group (Supplementary Table 6). While, the mycological eradication rate did not differ in NAC subgroups (Supplementary Table 5). The percentage of patients who achieved clinical cure but remained positive for Candida culture at TOC visit was also summarized by species infected at screening (Supplementary Table 7).
Fig. 3.
Results of clinical cure (a) and mycological eradication (b) in patients infected with C. albicans at test-of-cure visit
Safety
Overall, ibrexafungerp was well tolerated. At least one TEAE was reported by 63.1% and 42.3% of patients receiving ibrexafungerp and placebo, respectively (Table 4). All except one TEAEs were mild to moderate in severity. As for TRAE, 54.1% of patients receiving ibrexafungerp reported one or more TRAEs compared to 17.1% receiving placebo (Supplementary Table 8). By system organ class (SOC), TEAEs of gastrointestinal disorders were most frequently reported for ibrexafungerp (50.8% [124/244]), whereas infections and infestations were mostly reported for placebo (17.1% [21/123]). By preferred term (PT), the most common TEAE in the ibrexafungerp group was diarrhea (43.0%), followed by nausea (9.0%) and abdominal pain (4.1%). There existed consistency between TRAEs and TEAEs regarding the incidence and severity of SOC and PT.
Table 4.
Summary of treatment-emergent adverse events (TEAEs) reported in ≥ 2% of patients (safety set) a
| System organ class Preferred term |
Ibrexafungerp (N = 244) n (%) |
Placebo (N = 123) n (%) |
|---|---|---|
| Patient with ≥ 1 TEAE | 154 (63.1) | 52 (42.3) |
| Gastrointestinal disorders | 124 (50.8) | 13 (10.6) |
| Diarrhea | 105 (43.0) | 6 (4.9) |
| Nausea | 22 (9.0) | 0 |
| Abdominal pain | 10 (4.1) | 2 (1.6) |
| Upper abdominal pain | 7 (2.9) | 0 |
| Dry mouth | 2 (0.8) | 3 (2.4) |
| Gastroesophageal reflux disease | 0 | 3 (2.4) |
| Infections and infestations | 33 (13.5) | 21 (17.1) |
| Upper respiratory tract infection | 9 (3.7) | 3 (2.4) |
| Bacterial vulvovaginitis | 7 (2.9) | 10 (8.1) |
| Vaginal infection | 4 (1.6) | 5 (4.1) |
| Bacterial vaginosis | 3 (1.2) | 5 (4.1) |
| Investigations | 18 (7.4) | 10 (7.1) |
| Positive SARS-CoV-2 test | 8 (3.3) | 4 (3.3) |
| Nervous system disorders | 16 (6.6) | 11 (8.9) |
| Dizziness | 12 (4.9) | 7 (5.7) |
| Headache | 3 (1.2) | 3 (2.4) |
aAt each level of patient summarization, a patient is counted once if the patient reported ≥ 1 events
No TEAE led to study discontinuation. Two SAEs were separately reported by two patients in the placebo group, which were “papillary thyroid carcinoma” and “colorectal polyp, Barrett esophagus, esophageal papilloma, duodenal polyp”, respectively. Neither of the SAEs was treatment-related SAE. One pregnancy was reported in the ibrexafungerp group during the study, and the outcome was elective abortion.
Discussion
This was the first clinical trial to evaluate the efficacy and safety of ibrexafungerp in Chinese VVC patients. Our study demonstrated that ibrexafungerp was efficacious and well tolerated in Chinese VVC patients under the conditions of this study. The clinical cure (absence of vulvovaginal symptoms and signs) rates with ibrexafungerp vs. placebo in our study (51.0% vs. 25.6%, respectively) were similar to that of VANISH 303 study (50.5% vs. 28.6%) as well as US patients in VANISH 306 study (54.5% vs. 27.8%) [10, 11]. In the present study, ibrexafungerp demonstrated reproducible statistical superiority over placebo for VVC treatment in Chinese patients, in consistency with the result of similarly designed VANISH 303/306 study.
Our study revealed the sustained efficacy of ibrexafungerp. In the ibrexafungerp group, 80.3% of patients who achieved clinical cure at TOC visit (day 11 ± 3) remained asymptomatic at FU visit (day 25 ± 4). Whereas, several published studies showed that fluconazole under various regimens reported an 11%-20% decrease in sustained response from day 7–14 to day 28–35[15–17]. It may be attributed to the fungicidal activity of ibrexafungerp in comparison to the fungistatic activity of fluconazole [18, 19]. Therefore, it indicated that ibrexafungerp was superior to fluconazole in sustained therapeutic effect.
Based on the susceptibility tests for fluconazole against Candida species obtained at screening, our study also explored the efficacy of ibrexafungerp in both patients with fluconazole-susceptible and patients with non-susceptible C. albicans. In the ibrexafungerp group, it was observed a similar clinical cure rate between patients with fluconazole-susceptible and non-susceptible C. albicans (55.8% vs. 52.1%). Moreover, in the ibrexafungerp group, patients infected with fluconazole susceptible and non-susceptible C. albicans both had a high-mycological eradication rate (77.0% and 75%). The clinical benefits of ibrexafungerp for patients with fluconazole non-susceptible C. albicans were consistent with a preclinical study. In the preclinical study, MIC of the Candida isolates against ibrexafungerp was determined per broth microdilution method published by CLSI. A total of 178 Candida were tested, including 44 Candida isolates with known genotypic (FKS1 or FKS2 mutations), phenotypic, or clinical resistance to echinocandins. Ibrexafungerp MICs were low (≤ 0.5 μg/ml) for azole-resistant C. albicans, C. parapsilosis, and C. tropicalis isolates, which demonstrated in vitro fungicidal activity of ibrexafungerp against azole-resistant Candida species [20]. Nowadays, fluconazole-resistant C. albicans has been a growing and perplexing problem for treating VVC. One up-to-date literature published in 2022 collected 2000 Candida isolates from VVC patients in 23 hospitals to explore the sensitivity of Candida species to common antifungals in China. The result showed that the resistance rate for vulvovaginal C. albicans was significantly higher than that of non-C. albicans (73.41% [715/974] vs. 50.88% [115/226], P < 0.001). [6]. Based on the positive results of our study, ibrexafungerp would probably be an alternative option for treatment of patients with fluconazole non-susceptible C. albicans in China.
Among patients with NAC in the ibrexafungerp group, the clinical cure rate was 43.6%, while the mycological eradication rate was just 12.8% at TOC visit. Of these, 67.6% of patients with positive culture for NAC were asymptomatic. The inconsistency between clinical cure and mycological eradication rate may be explained by the Candida colonization in vagina. A previous study of Kennedy et al. showed that at least 50% of women with positive cultures for NAC might be minimally symptomatic or had no symptoms, while 80–85% of patients with positive cultures for C. albicans would be likely to have symptoms [21], which was consistent with our study. Moreover, clinical practice guidelines recommended that asymptomatic patients with mere mycological persistence required no further therapy [3, 22].
According to pharmaceutical industry guidance issued by the Food and Drug Administration in 2019, our study chose placebo rather than fluconazole as a comparator using a superiority design and adopted clinical cure (i.e., the complete resolution of signs and symptoms of VVC without need for further antifungal treatment before or at TOC visit) as the primary endpoint. Nevertheless, the efficacy of single-dose fluconazole and single-day ibrexafungerp was compared in a US phase II study (DOVE), in which the clinical cure rate in ibrexafungerp group was similar with that of fluconazole group on day 10 (51.9% vs. 58.3%) but higher than that of fluconazole group on day 25 (70.4% vs. 50.0%)[23]. Moreover, a randomized, double-blind phase III trial of a new antifungal agent in China adopted the same definition of clinical cure (VSS score = 0) and mITT population. The study found that in the fluconazole group, the percentage of subjects reaching clinical cure on day 14 in the mITT population and subgroup of C. albicans was 50.31% and 53.72%, respectively, which is similar to those of ibrexafungerp in our study (51.0% for mITT population; 55.8% for a subgroup of C. albicans on day 11 ± 3) [24].
Ibrexafungerp was well tolerable in China’s VVC patients when administered as a 300 mg oral tablet BID for 1 day. Although, a higher percentage of patients receiving ibrexafungerp (54.1%) experienced at least one TRAE compared with those receiving placebo (17.1%), most TRAEs were mild to moderate and recovered without any intervention. Consistent with VANISH 303/306 study, the most common TRAEs were gastrointestinal disorders. The incidence of gastrointestinal disorders in Chinese patients was similar to that of U.S. patients in VANISH 303/306 study (50.8% vs. 42.9%). Chinese patients experienced a higher incidence of diarrhea than U.S. patients (43.0% vs. 23.9%), but fewer nausea and abdominal pain (9.0% each) than US patients (16.8% and 14.8%, respectively) [25]. It was speculated that the differences in dietary habits between Chinese and US patients contributed to the various gastrointestinal reactions. All three clinical trials demonstrated the good safety of ibrexafungerp.
While, some limitations existed in our study the clinical efficacy of ibrexafungerp in obese or diabetic patients could not be determined in the subgroup analyses due to the small sample size. Therefore, future research on ibrexafungerp was warranted, especially its effectiveness in specific patients.
Conclusion
In conclusion, ibrexafungerp is a novel, oral antifungal with a statistically superior efficacy to placebo. Moreover, ibrexafungerp was well tolerated in Chinese patients and most TRAEs were gastrointestinal disorders that were mild to moderate in nature. Given the significant clinical benefits and good safety of ibrexafungerp, it would probably be a new option for Chinese VVC patients in the future.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We appreciate all the patients and their families, the investigators and investigational staff who participated in this study. We thank Jiayang Song and Peng Zhang for carefully analyzing and reviewing the data and also thank Kaimeng Kong and Wensu Yu for medical writing assistance during manuscript preparation. This study was funded by the Jiangsu Hansoh Pharmaceutical Group Co., Ltd.
Author contributions
Qinping Liao, Xiaoqian Wang, Wenying Wang, Jingjing Li, Ruifang An, Lihong Chen, Jiajing Lin, Dabao Xu, Jin Qiu, Weihua Song, Mijiti Patiman, Hongjie Ruan, Gang Wang, Fengxia Xue, Xu Wang, Xiaowan Luo, Qi Ruan, Ling Shi, Chun Zhang, Lina Hu, Shijin Wang, Hong Shi, Xiaoli Wang, Songling Zhang, Yingxiong Li, Jing Lu, Baojin Wang, Hongyan Xu, Hong Ye, Bei Zhang, Chunlian Zhang, and Sumin Qian contributed to the acquisition of data. Chuan Li, Peng Zhang and Jiayang Song contributed to statistical analysis. Xiaoqian Wang, Qinping Liao, Wen Jia, Wensu Yu, and Kaimeng Kong contributed to drafting the manuscript. All authors critically revised it for important intellectual content. All authors read and approved the final manuscript.
Funding
This study was funded by the Jiangsu Hansoh Pharmaceutical Group Co., Ltd.
Data availability
The source data that support the findings of this study are available from Jiangsu Hansoh Pharmaceutical Group Co., Ltd. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the corresponding author (Qinping Liao) with the permission of Jiangsu Hansoh Pharmaceutical Group Co., Ltd.
Declarations
Conflict of interest
Qiong Wu, Wen Jia and Chuan Li are the employees of Jiangsu Hansoh Pharmaceutical Group Co., Ltd. Other authors have nothing to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki, Good Clinical Practice, and the current International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Guidelines. The study was also approved by the ethics committees of the study sites.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Mendling W, Brasch J. Guideline vulvovaginal candidosis (2010) of the German Society for Gynecology and Obstetrics, the Working Group for Infections and Infectimmunology in Gynecology and Obstetrics, the German Society of Dermatology, the Board of German Dermatologists and the German Speaking Mycological Society. Mycoses. 2012;55(Suppl 3):1–13. 10.1111/j.1439-0507.2012.02185.x. [DOI] [PubMed] [Google Scholar]
- 2.Martin Lopez JE. Candidiasis (vulvovaginal). BMJ Clin Evid. 2015;2015:0815. [PMC free article] [PubMed] [Google Scholar]
- 3.Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, Reno H, Zenilman JM, Bolan GA. Sexually transmitted diseases treatment guideline 2021. MMWR Recomm Rep. 2021;70(4):1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang FJ, Zhang D, Liu ZH, Wu WX, Bai HH, Dong HY. Species distribution and in vitro antifungal susceptibility of vulvovaginal candida isolates in China. Chin Med J. 2016;129(10):1161–5. 10.4103/0366-6999.181964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Bijie H, Dzierzanowska D, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997–2007: 10.5-year analysis of susceptibilities of noncandidal yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol. 2009;47(1):117–23. 10.1128/jcm.01747-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan LY, Liu ZH, Bai XN, Zong X. Analysis of resistance and sensitivity of 1 200 strains of vulvovaginal candidiasis in China to five common antifungal drugs. Zhonghua Fu Chan Ke Za Zhi. 2022;57(8):601–7. 10.3760/cma.j.cn112141-20220211-00076. [DOI] [PubMed] [Google Scholar]
- 7.Sobel JD, Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin Pharmacother. 2018;19(9):971–7. 10.1080/14656566.2018.1476490. [DOI] [PubMed] [Google Scholar]
- 8.Apgar JM, Wilkening RR, Parker DL Jr, Meng D, Wildonger KJ, Sperbeck D, et al. Ibrexafungerp: an orally active β-1,3-glucan synthesis inhibitor. Bioorg Med Chem Lett. 2021;32: 127661. 10.1016/j.bmcl.2020.127661. [DOI] [PubMed] [Google Scholar]
- 9.Azie N, Angulo D, Dehn B, Sobel JD. Oral Ibrexafungerp: an investigational agent for the treatment of vulvovaginal candidiasis. Expert Opin Investig Drugs. 2020;29(9):893–900. 10.1080/13543784.2020.1791820. [DOI] [PubMed] [Google Scholar]
- 10.Schwebke JR, Sobel R, Gersten JK, Sussman SA, Lederman SN, Jacobs MA, et al. Ibrexafungerp versus placebo for vulvovaginal candidiasis treatment: a phase 3, randomized, controlled superiority trial (VANISH 303). Clin Infect Dis. 2022;74(11):1979–85. 10.1093/cid/ciab750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sobel R, Nyirjesy P, Ghannoum MA, Delchev DA, Azie NE, Angulo D, et al. Efficacy and safety of oral ibrexafungerp for the treatment of acute vulvovaginal candidiasis: a global phase 3, randomised, placebo-controlled superiority study (VANISH 306). BJOG. 2022;129(3):412–20. 10.1111/1471-0528.16972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Administration UFaD. BREXAFEMME® (ibrexafungerp tablets), for oral use. (2022). https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214900s002lbl.pdf. Accessed 02 Dec 2022.
- 13.Institute CaLS. Epidemiological cutoff values for antifungal susceptibility testing. 3rd ed. Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 14.Institute CaLS. In performance standards for antifungal susceptibility testing of yeasts. 1st ed. Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 15.Li T, Zhu Y, Fan S, Liu X, Xu H, Liang Y. A randomized clinical trial of the efficacy and safety of terconazole vaginal suppository versus oral fluconazole for treating severe vulvovaginal candidiasis. Med Mycol. 2015;53(5):455–61. 10.1093/mmy/myv017. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X, Li T, Fan S, Zhu Y, Liu X, Guo X, et al. The efficacy and safety of clotrimazole vaginal tablet vs oral fluconazole in treating severe vulvovaginal candidiasis. Mycoses. 2016;59(7):419–28. 10.1111/myc.12485. [DOI] [PubMed] [Google Scholar]
- 17.Sobel JD, Kapernick PS, Zervos M, Reed BD, Hooton T, Soper D, et al. Treatment of complicated Candida vaginitis: comparison of single and sequential doses of fluconazole. Am J Obstet Gynecol. 2001;185(2):363–9. 10.1067/mob.2001.115116. [DOI] [PubMed] [Google Scholar]
- 18.Administration UFaD. DIFLUCAN® (Fluconazole Tablets) (Fluconazole for Oral Suspension). (2023). https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/019949s074,020090s055lbl.pdf. Accessed 14 Jul 2023.
- 19.Sucher AJ, Thai A, Tran C, Mantena N, Noronha A, Chahine EB. Ibrexafungerp: a new triterpenoid antifungal. Am J Health Syst Pharm. 2022;79(24):2208–21. 10.1093/ajhp/zxac256. [DOI] [PubMed] [Google Scholar]
- 20.Schell WA, Jones AM, Borroto-Esoda K, Alexander BD. Antifungal activity of SCY-078 and standard antifungal Agents against 178 clinical isolates of resistant and susceptible Candida species. Antimicrob Agents Chemother. 2017. 10.1128/aac.01102-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy MA, Sobel JD. Vulvovaginal candidiasis caused by non-Albicans candida species: new insights. Curr Infect Dis Rep. 2010;12(6):465–70. 10.1007/s11908-010-0137-9. [DOI] [PubMed] [Google Scholar]
- 22.van Schalkwyk J, Yudin MH, Infectious DC. Vulvovaginitis: screening for and management of trichomoniasis vulvovaginal candidiasis and bacterial vaginosis. J Obstet Gynaecol Can. 2015;37(3):266–74. 10.1016/S1701-2163(15)30316-9. [DOI] [PubMed] [Google Scholar]
- 23.Nyirjesy P, Schwebke JR, Angulo DA, Harriott IA, Azie NE, Sobel JD. Phase 2 randomized study of oral ibrexafungerp vs fluconazole in vulvovaginal candidiasis. Clin Infect Dis. 2021. 10.1093/cid/ciab841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Chen L, Ruan H, Xiong Z, Wang W, Qiu J, et al. Oteseconazole versus fluconazole for the treatment of severe vulvovaginal candidiasis: a multicenter, randomized, double-blinded, phase 3 trial. Antimicrob Agents Chemother. 2023;68:e0077823. 10.1128/aac.00778-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Administration UFaD. NDA Multi-disciplinary Review and Evaluation - NDA 214900. (2021). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214900Orig1s000MultidisciplineR.pdf. Accessed 30 Jun 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The source data that support the findings of this study are available from Jiangsu Hansoh Pharmaceutical Group Co., Ltd. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the corresponding author (Qinping Liao) with the permission of Jiangsu Hansoh Pharmaceutical Group Co., Ltd.