Abstract
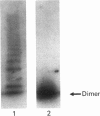
We have established that a recombinant von Willebrand Factor (vWF) mutant (vWFdelpro) that lacks the propolypeptide, in contrast with mature wild-type vWF, with which it is identical in terms of primary amino acid sequence, is not able to form a complex with Factor VIII. Wild-type vWF (flvWF) and vWFdelpro were expressed in AtT-20 cells. Under the culture conditions employed, completely processed multimerized flvWF and dimeric vWFdelpro were secreted into the medium. FlvWF and vWFdelpro were compared for their Factor VIII-binding properties in two distinct assay systems. In a direct binding assay, purified human Factor VIII was shown to bind to flvWF that had been immobilized on the surface of microtitre wells by using an anti-vWF monoclonal antibody. In contrast, Factor VIII did not bind to immobilized vWFdelpro. In a competition assay, fluid-phase flvWF appeared to inhibit efficiently the binding of Factor VIII to immobilized vWF isolated from plasma, whereas vWFdelpro did not influence Factor VIII binding. From these observations, it is argued that the pro-polypeptide serves an essential role in the post-translational processes that lead to the expression of a functional Factor VIII-binding site on the mature vWF subunit.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. O., Forsman N., Huang K., Larsen K., Lundin A., Pavlu B., Sandberg H., Sewerin K., Smart J. Isolation and characterization of human factor VIII: molecular forms in commercial factor VIII concentrate, cryoprecipitate, and plasma. Proc Natl Acad Sci U S A. 1986 May;83(9):2979–2983. doi: 10.1073/pnas.83.9.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahou W. F., Ginsburg D., Sikkink R., Litwiller R., Fass D. N. A monoclonal antibody to von Willebrand factor (vWF) inhibits factor VIII binding. Localization of its antigenic determinant to a nonadecapeptide at the amino terminus of the mature vWF polypeptide. J Clin Invest. 1989 Jul;84(1):56–61. doi: 10.1172/JCI114169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthron D. T., Handin R. I., Kaufman R. J., Wasley L. C., Orr E. C., Mitsock L. M., Ewenstein B., Loscalzo J., Ginsburg D., Orkin S. H. Structure of pre-pro-von Willebrand factor and its expression in heterologous cells. Nature. 1986 Nov 20;324(6094):270–273. doi: 10.1038/324270a0. [DOI] [PubMed] [Google Scholar]
- Brinkhous K. M., Sandberg H., Garris J. B., Mattsson C., Palm M., Griggs T., Read M. S. Purified human factor VIII procoagulant protein: comparative hemostatic response after infusions into hemophilic and von Willebrand disease dogs. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8752–8756. doi: 10.1073/pnas.82.24.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts R. B., Paskell S. L., Elgee S. K. Disulfide bonds and the quaternary structure of factor VIII/von Willebrand factor. J Clin Invest. 1978 Sep;62(3):702–709. doi: 10.1172/JCI109178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D., Rodriguez H., Vehar G. A. Proteolytic processing of human factor VIII. Correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry. 1986 Jan 28;25(2):505–512. doi: 10.1021/bi00350a035. [DOI] [PubMed] [Google Scholar]
- Fay P. J., Anderson M. T., Chavin S. I., Marder V. J. The size of human factor VIII heterodimers and the effects produced by thrombin. Biochim Biophys Acta. 1986 Jun 23;871(3):268–278. doi: 10.1016/0167-4838(86)90208-6. [DOI] [PubMed] [Google Scholar]
- Foster P. A., Fulcher C. A., Houghten R. A., Zimmerman T. S. An immunogenic region within residues Val1670-Glu1684 of the factor VIII light chain induces antibodies which inhibit binding of factor VIII to von Willebrand factor. J Biol Chem. 1988 Apr 15;263(11):5230–5234. [PubMed] [Google Scholar]
- Foster P. A., Fulcher C. A., Marti T., Titani K., Zimmerman T. S. A major factor VIII binding domain resides within the amino-terminal 272 amino acid residues of von Willebrand factor. J Biol Chem. 1987 Jun 25;262(18):8443–8446. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hoyer L. W., Shainoff J. R. Factor VIII-related protein circulates in normal human plasma as high molecular weight multimers. Blood. 1980 Jun;55(6):1056–1059. [PubMed] [Google Scholar]
- Kane W. H., Davie E. W. Blood coagulation factors V and VIII: structural and functional similarities and their relationship to hemorrhagic and thrombotic disorders. Blood. 1988 Mar;71(3):539–555. [PubMed] [Google Scholar]
- Leyte A., Mertens K., Distel B., Evers R. F., De Keyzer-Nellen M. J., Groenen-Van Dooren M. M., De Bruin J., Pannekoek H., Van Mourik J. A., Verbeet M. P. Inhibition of human coagulation factor VIII by monoclonal antibodies. Mapping of functional epitopes with the use of recombinant factor VIII fragments. Biochem J. 1989 Oct 1;263(1):187–194. doi: 10.1042/bj2630187. [DOI] [PMC free article] [PubMed] [Google Scholar]
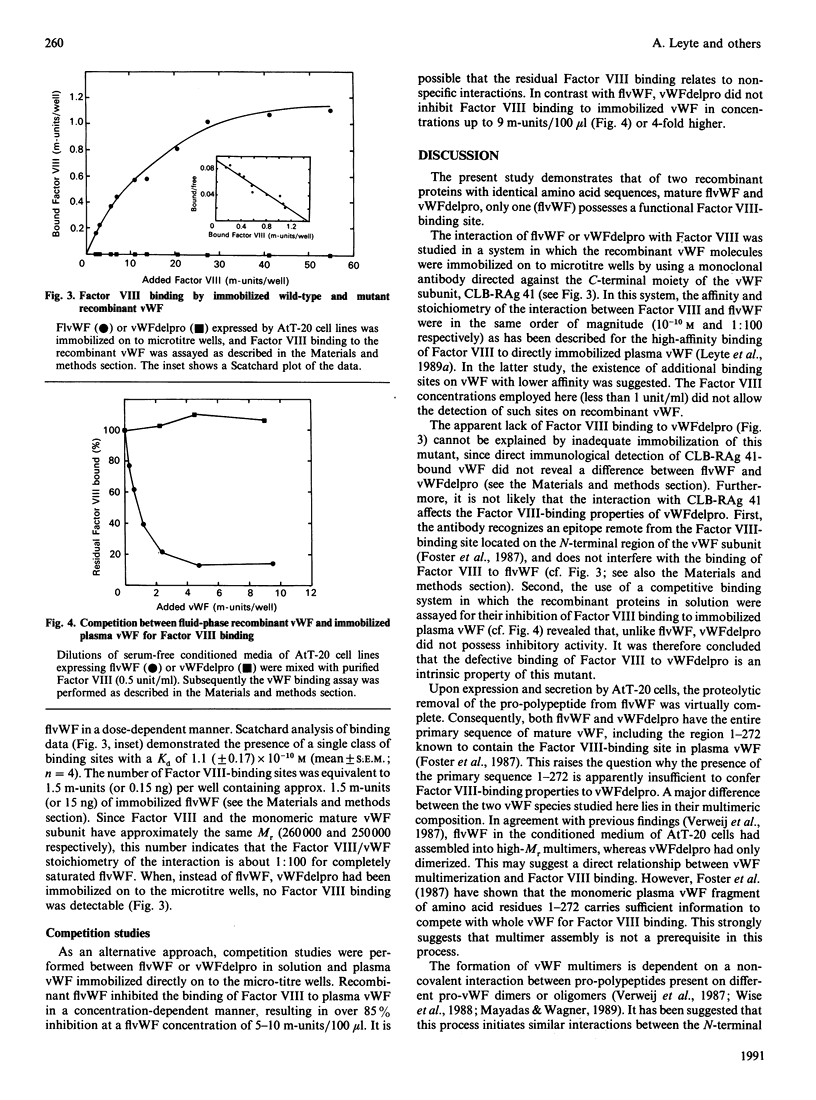
- Leyte A., Verbeet M. P., Brodniewicz-Proba T., Van Mourik J. A., Mertens K. The interaction between human blood-coagulation factor VIII and von Willebrand factor. Characterization of a high-affinity binding site on factor VIII. Biochem J. 1989 Feb 1;257(3):679–683. doi: 10.1042/bj2570679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lollar P., Hill-Eubanks D. C., Parker C. G. Association of the factor VIII light chain with von Willebrand factor. J Biol Chem. 1988 Jul 25;263(21):10451–10455. [PubMed] [Google Scholar]
- Lynch D. C., Williams R., Zimmerman T. S., Kirby E. P., Livingston D. M. Biosynthesis of the subunits of factor VIIIR by bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2738–2742. doi: 10.1073/pnas.80.9.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M. E., van Zonneveld A. J., Pannekoek H. Functional analysis of the human tissue-type plasminogen activator protein: the light chain. Gene. 1986;42(1):59–67. doi: 10.1016/0378-1119(86)90150-2. [DOI] [PubMed] [Google Scholar]
- Marti T., Rösselet S. J., Titani K., Walsh K. A. Identification of disulfide-bridged substructures within human von Willebrand factor. Biochemistry. 1987 Dec 15;26(25):8099–8109. doi: 10.1021/bi00399a013. [DOI] [PubMed] [Google Scholar]
- Mayadas T. N., Wagner D. D. In vitro multimerization of von Willebrand factor is triggered by low pH. Importance of the propolypeptide and free sulfhydryls. J Biol Chem. 1989 Aug 15;264(23):13497–13503. [PubMed] [Google Scholar]
- Mazurier C., Dieval J., Jorieux S., Delobel J., Goudemand M. A new von Willebrand factor (vWF) defect in a patient with factor VIII (FVIII) deficiency but with normal levels and multimeric patterns of both plasma and platelet vWF. Characterization of abnormal vWF/FVIII interaction. Blood. 1990 Jan 1;75(1):20–26. [PubMed] [Google Scholar]
- Mertens K., van Wijngaarden A., Bertina R. M. The role of factor VIII in the activation of human blood coagulation factor X by activated factor IX. Thromb Haemost. 1985 Oct 30;54(3):654–660. [PubMed] [Google Scholar]
- Nishino M., Girma J. P., Rothschild C., Fressinaud E., Meyer D. New variant of von Willebrand disease with defective binding to factor VIII. Blood. 1989 Oct;74(5):1591–1599. [PubMed] [Google Scholar]
- Over J., Sixma J. J., Bouma B. N., Bolhuis P. A., Vlooswijk R. A., Beeser-Visser N. H. Survival of iodine-125-labeled factor VIII in patients with von Willebrand's disease. J Lab Clin Med. 1981 Mar;97(3):332–344. [PubMed] [Google Scholar]
- Piétu G., Ribba A. S., Meulien P., Meyer D. Localization within the 106 N-terminal amino acids of von Willebrand factor (vWF) of the epitope corresponding to a monoclonal antibody which inhibits vWF binding to factor VIII. Biochem Biophys Res Commun. 1989 Aug 30;163(1):618–626. doi: 10.1016/0006-291x(89)92182-7. [DOI] [PubMed] [Google Scholar]
- Reinders J. H., Vervoorn R. C., Verweij C. L., van Mourik J. A., de Groot P. G. Perturbation of cultured human vascular endothelial cells by phorbol ester or thrombin alters the cellular von Willebrand factor distribution. J Cell Physiol. 1987 Oct;133(1):79–87. doi: 10.1002/jcp.1041330110. [DOI] [PubMed] [Google Scholar]
- Rotblat F., O'Brien D. P., O'Brien F. J., Goodall A. H., Tuddenham E. G. Purification of human factor VIII:C and its characterization by Western blotting using monoclonal antibodies. Biochemistry. 1985 Jul 30;24(16):4294–4300. doi: 10.1021/bi00337a007. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981 Jun;57(6):1140–1143. [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. Variant von Willebrand's disease: characterization of two subtypes by analysis of multimeric composition of factor VIII/von Willebrand factor in plasma and platelets. J Clin Invest. 1980 Jun;65(6):1318–1325. doi: 10.1172/JCI109795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler J. E., Shelton-Inloes B. B., Sorace J. M., Harlan J. M., Titani K., Davie E. W. Cloning and characterization of two cDNAs coding for human von Willebrand factor. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6394–6398. doi: 10.1073/pnas.82.19.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakariassen K. S., Bolhuis P. A., Sixma J. J. Human blood platelet adhesion to artery subendothelium is mediated by factor VIII-Von Willebrand factor bound to the subendothelium. Nature. 1979 Jun 14;279(5714):636–638. doi: 10.1038/279636a0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stel H. V., Sakariassen K. S., Scholte B. J., Veerman E. C., van der Kwast T. H., de Groot P. G., Sixma J. J., van Mourik J. A. Characterization of 25 monoclonal antibodies to factor VIII-von Willebrand factor: relationship between ristocetin-induced platelet aggregation and platelet adherence to subendothelium. Blood. 1984 Jun;63(6):1408–1415. [PubMed] [Google Scholar]
- Titani K., Kumar S., Takio K., Ericsson L. H., Wade R. D., Ashida K., Walsh K. A., Chopek M. W., Sadler J. E., Fujikawa K. Amino acid sequence of human von Willebrand factor. Biochemistry. 1986 Jun 3;25(11):3171–3184. doi: 10.1021/bi00359a015. [DOI] [PubMed] [Google Scholar]
- Toole J. J., Knopf J. L., Wozney J. M., Sultzman L. A., Buecker J. L., Pittman D. D., Kaufman R. J., Brown E., Shoemaker C., Orr E. C. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984 Nov 22;312(5992):342–347. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- Tschopp T. B., Weiss H. J., Baumgartner H. R. Decreased adhesion of platelets to subendothelium in von Willebrand's disease. J Lab Clin Med. 1974 Feb;83(2):296–300. [PubMed] [Google Scholar]
- Tuddenham E. G., Lane R. S., Rotblat F., Johnson A. J., Snape T. J., Middleton S., Kernoff P. B. Response to infusions of polyelectrolyte fractionated human factor VIII concentrate in human haemophilia A and von Willebrand's disease. Br J Haematol. 1982 Oct;52(2):259–267. doi: 10.1111/j.1365-2141.1982.tb03888.x. [DOI] [PubMed] [Google Scholar]
- Vehar G. A., Davie E. W. Preparation and properties of bovine factor VIII (antihemophilic factor). Biochemistry. 1980 Feb 5;19(3):401–410. doi: 10.1021/bi00544a001. [DOI] [PubMed] [Google Scholar]
- Vehar G. A., Keyt B., Eaton D., Rodriguez H., O'Brien D. P., Rotblat F., Oppermann H., Keck R., Wood W. I., Harkins R. N. Structure of human factor VIII. Nature. 1984 Nov 22;312(5992):337–342. doi: 10.1038/312337a0. [DOI] [PubMed] [Google Scholar]
- Verweij C. L., Diergaarde P. J., Hart M., Pannekoek H. Full-length von Willebrand factor (vWF) cDNA encodes a highly repetitive protein considerably larger than the mature vWF subunit. EMBO J. 1986 Aug;5(8):1839–1847. doi: 10.1002/j.1460-2075.1986.tb04435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij C. L., Hart M., Pannekoek H. Expression of variant von Willebrand factor (vWF) cDNA in heterologous cells: requirement of the pro-polypeptide in vWF multimer formation. EMBO J. 1987 Oct;6(10):2885–2890. doi: 10.1002/j.1460-2075.1987.tb02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij C. L., de Vries C. J., Distel B., van Zonneveld A. J., van Kessel A. G., van Mourik J. A., Pannekoek H. Construction of cDNA coding for human von Willebrand factor using antibody probes for colony-screening and mapping of the chromosomal gene. Nucleic Acids Res. 1985 Jul 11;13(13):4699–4717. doi: 10.1093/nar/13.13.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorberg J., Fontijn R., van Mourik J. A., Pannekoek H. Domains involved in multimer assembly of von willebrand factor (vWF): multimerization is independent of dimerization. EMBO J. 1990 Mar;9(3):797–803. doi: 10.1002/j.1460-2075.1990.tb08176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Marder V. J. Biosynthesis of von Willebrand protein by human endothelial cells: processing steps and their intracellular localization. J Cell Biol. 1984 Dec;99(6):2123–2130. doi: 10.1083/jcb.99.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. J., Pittman D. D., Handin R. I., Kaufman R. J., Orkin S. H. The propeptide of von Willebrand factor independently mediates the assembly of von Willebrand multimers. Cell. 1988 Jan 29;52(2):229–236. doi: 10.1016/0092-8674(88)90511-9. [DOI] [PubMed] [Google Scholar]
- van Dieijen G., Tans G., Rosing J., Hemker H. C. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J Biol Chem. 1981 Apr 10;256(7):3433–3442. [PubMed] [Google Scholar]
- van Mourik J. A., Bolhuis P. A. Dispersity of human factor VIII--Von Willebrand factor. Thromb Res. 1978 Jul;13(1):15–24. doi: 10.1016/0049-3848(78)90105-6. [DOI] [PubMed] [Google Scholar]