Abstract
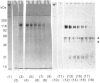
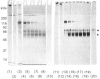
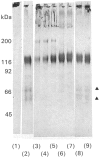
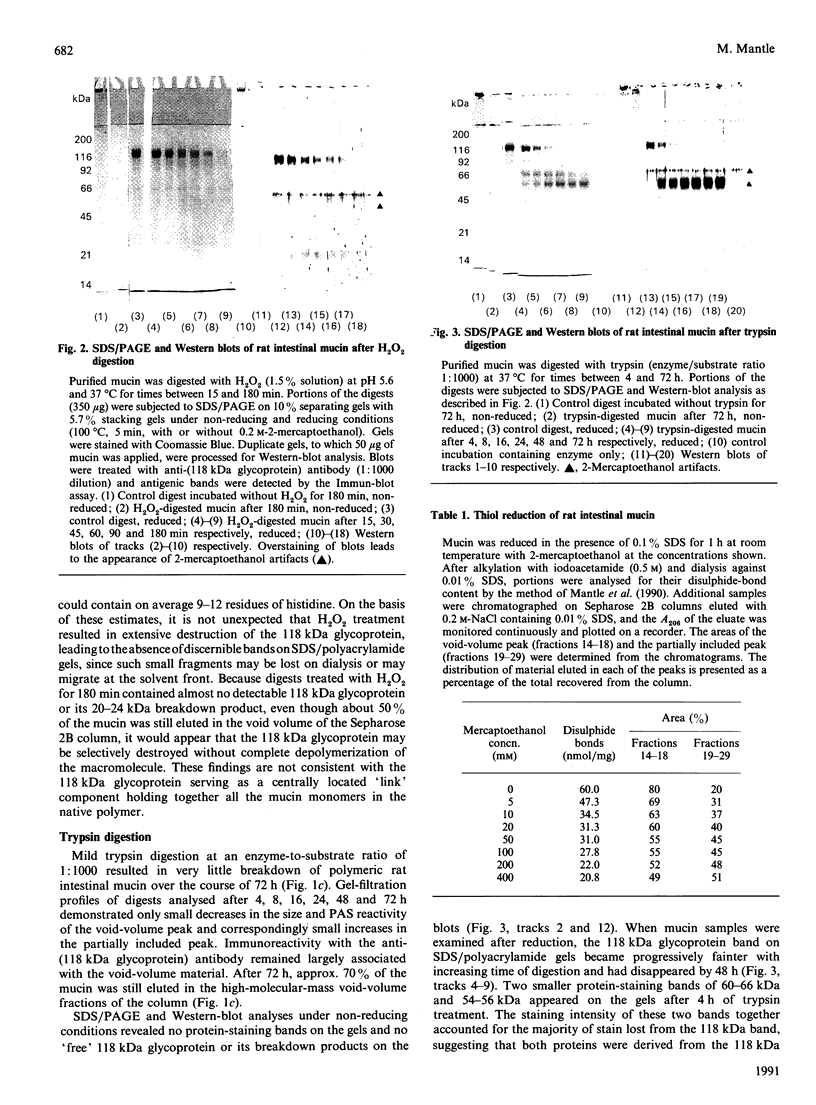
The role of the disulphide-bound 118 kDa glycoprotein of rat intestinal mucin is unknown, although it has been proposed to serve as a 'link' component for the mucin monomers. The present studies investigated release or destruction of the 118 kDa glycoprotein (monitored by gel electrophoresis and Western-blot analysis) during progressive breakdown of the mucin polymer (assessed by Sepharose 2B chromatography). H2O2 gradually destroyed the 118 kDa glycoprotein and dissociated the mucin polymer into components of similar size to the monomers. After 3 h, mucin samples contained almost no 118 kDa glycoprotein or its breakdown products, but 50% of the mucin was still eluted in the void volume of a Sepharose 2B column. Although mild trypsinolysis had little effect on the Sepharose 2B elution profile of the mucin, the 118 kDa glycoprotein was completely cleaved into 54-56 kDa and 60-66 kDa fragments which remained disulphide-bound to the high-molecular-mass mucin. Increasing levels of thiol reduction resulted in progressive loss of disulphide bonds, release of the 118 kDa glycoprotein and depolymerization of the mucin. Although approx. 40% of the mucin in partially reduced samples was recovered in the Sepharose 2B void volume, this material contained no 118 kDa glycoprotein and apparently consisted of disulphide-bound mucin monomers. Thus the 118 kDa glycoprotein may be destroyed by H2O2, extensively cleaved by trypsin or released by reduction without completely dissociating the mucin into monomers. Therefore the 118 kDa glycoprotein may not function as a 'link' component for all of the mucin monomers in the native polymer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaplin M. F. A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Anal Biochem. 1982 Jul 1;123(2):336–341. doi: 10.1016/0003-2697(82)90455-9. [DOI] [PubMed] [Google Scholar]
- Cooper B., Creeth J. M., Donald A. S. Studies of the limited degradation of mucus glycoproteins. The mechanism of the peroxide reaction. Biochem J. 1985 Jun 15;228(3):615–626. doi: 10.1042/bj2280615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeth J. M., Cooper B., Donald A. S., Clamp J. R. Studies of the limited degradation of mucus glycoproteins. The effect of dilute hydrogen peroxide. Biochem J. 1983 May 1;211(2):323–332. doi: 10.1042/bj2110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiffert H., Quentin E., Decker J., Hillemeir S., Hufschmidt M., Klingmüller D., Weber M. H., Hilschmann N. Die Primärstruktur der menschlichen freien Sekretkomponente und die Anordnung der Disulfidbrücken. Hoppe Seylers Z Physiol Chem. 1984 Dec;365(12):1489–1495. [PubMed] [Google Scholar]
- Fahim R. E., Forstner G. G., Forstner J. F. Heterogeneity of rat goblet-cell mucin before and after reduction. Biochem J. 1983 Jan 1;209(1):117–124. doi: 10.1042/bj2090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim R. E., Forstner G. G., Forstner J. F. Structural and compositional differences between intracellular and secreted mucin of rat small intestine. Biochem J. 1987 Dec 1;248(2):389–396. doi: 10.1042/bj2480389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim R. E., Specian R. D., Forstner G. G., Forstner J. F. Characterization and localization of the putative 'link' component in rat small-intestinal mucin. Biochem J. 1987 May 1;243(3):631–640. doi: 10.1042/bj2430631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Hull S. R., Sheng Z., Vanderpuye O., David C., Carraway K. L. Isolation and partial characterization of ascites sialoglycoprotein-2 of the cell surface sialomucin complex of 13762 rat mammary adenocarcinoma cells. Biochem J. 1990 Jan 1;265(1):121–129. doi: 10.1042/bj2650121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Antigenic and structural features of goblet-cell mucin of human small intestine. Biochem J. 1984 Jan 1;217(1):159–167. doi: 10.1042/bj2170159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Biochemical characterization of the component parts of intestinal mucin from patients with cystic fibrosis. Biochem J. 1984 Dec 1;224(2):345–354. doi: 10.1042/bj2240345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Mantle D., Allen A. Polymeric structure of pig small-intestinal mucus glycoprotein. Dissociation by proteolysis or by reduction of disulphide bridges. Biochem J. 1981 Apr 1;195(1):277–285. doi: 10.1042/bj1950277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Stewart G. Intestinal mucins from normal subjects and patients with cystic fibrosis. Variable contents of the disulphide-bound 118 kDa glycoprotein and different reactivities with an anti-(118 kDa glycoprotein) antibody. Biochem J. 1989 Apr 1;259(1):243–253. doi: 10.1042/bj2590243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Stewart G., Zayas G., King M. The disulphide-bond content and rheological properties of intestinal mucins from normal subjects and patients with cystic fibrosis. Biochem J. 1990 Mar 1;266(2):597–604. [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Thakore E. Rabbit intestinal and colonic mucins: isolation, partial characterization, and measurement of secretion using an enzyme-linked immunoassay. Biochem Cell Biol. 1988 Oct;66(10):1045–1054. doi: 10.1139/o88-121. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Friedlander M., Blobel G. The receptor for transepithelial transport of IgA and IgM contains multiple immunoglobulin-like domains. Nature. 1984 Mar 1;308(5954):37–43. doi: 10.1038/308037a0. [DOI] [PubMed] [Google Scholar]
- Pearson J. P., Allen A., Parry S. A 70000-molecular-weight protein isolated from purified pig gastric mucus glycoprotein by reduction of disulphide bridges and its implication in the polymeric structure. Biochem J. 1981 Jul 1;197(1):155–162. doi: 10.1042/bj1970155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringler N. J., Selvakumar R., Woodward H. D., Bhavanandan V. P., Davidson E. A. Protein components of human tracheobronchial mucin: partial characterization of a closely associated 65-kilodalton protein. Biochemistry. 1988 Oct 18;27(21):8056–8063. doi: 10.1021/bi00421a013. [DOI] [PubMed] [Google Scholar]
- Ringler N. J., Selvakumar R., Woodward H. D., Simet I. M., Bhavanandan V. P., Davidson E. A. Structure of canine tracheobronchial mucin glycoprotein. Biochemistry. 1987 Aug 25;26(17):5322–5328. doi: 10.1021/bi00391a016. [DOI] [PubMed] [Google Scholar]
- Roberton A. M., Mantle M., Fahim R. E., Specian R. D., Bennick A., Kawagishi S., Sherman P., Forstner J. F. The putative 'link' glycopeptide associated with mucus glycoproteins. Composition and properties of preparations from the gastrointestinal tracts of several mammals. Biochem J. 1989 Jul 15;261(2):637–647. doi: 10.1042/bj2610637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z. Q., Hull S. R., Carraway K. L. Biosynthesis of the cell surface sialomucin complex of ascites 13762 rat mammary adenocarcinoma cells from a high molecular weight precursor. J Biol Chem. 1990 May 25;265(15):8505–8510. [PubMed] [Google Scholar]
- Tomasi T. B. Regulation of the mucosal IGA response--an overview. Immunol Invest. 1989 Jan-May;18(1-4):1–15. doi: 10.3109/08820138909112223. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]