Abstract
Activation of phospholipase A2 (PLA2) in response to external stimuli may play a pivotal role in signal-transduction pathways via the generation of important cellular intermediates, including prostaglandins. Epidermal growth factor (EGF) has been shown to modulate prostaglandin production, possibly via direct activation of PLA2 or indirectly via interaction with a PLA2-modifying protein such as lipocortin I. We have investigated these pathways with two CHO cell-lines, one (CHOwt) transfected with the full-length human EGF receptor and the second (CHO 11) with a deletion mutant, delta 990, that has lost the autophosphorylation sites and part of the internalization domain. CHOwt cells responded to EGF with a rapid rise in lysophosphatidylcholine and arachidonic acid release concomitant with an increase in prostaglandin production. However, in the non-internalizing CHO 11 cells no such activation of PLA2 was observed. This was not due to an intrinsic lack of PLA2 in these cells, as PLA2 activation was shown on melittin addition, nor was this difference due to a defect in intracellular pathways, as arachidonic acid was released from both cell types by Ca2+ and protein kinase C modulators. However, only in CHOwt cells were these responses potentiated by concomitant addition of EGF. Thus the cytoplasmic subdomain of the EGF receptor, containing the major sites of autophosphorylation and the internalization domain, seems to be involved in the activation of PLA2 by EGF. In addition, we have shown that phosphorylation of lipocortin I is unlikely to play a role in PLA2 activation. In CHOwt cells and a positive control cell line, A431, activation of PLA2 was complete by 10 min, at which time there was no evidence of lipocortin I phosphorylation.
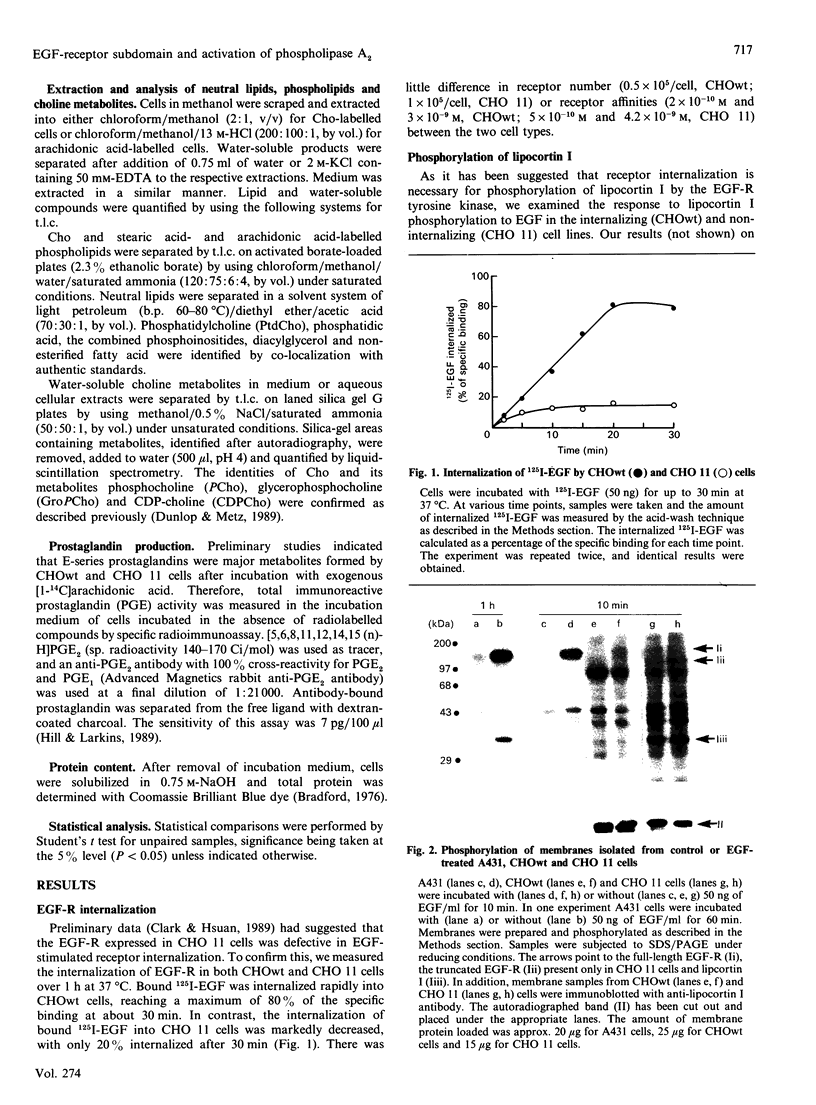
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyagi T., Suya H., Kato N., Nemoto O., Kobayashi H., Miura Y. Epidermal growth factor stimulates release of arachidonic acid in pig epidermis. J Invest Dermatol. 1985 Mar;84(3):168–171. doi: 10.1111/1523-1747.ep12264690. [DOI] [PubMed] [Google Scholar]
- Argiolas A., Pisano J. J. Facilitation of phospholipase A2 activity by mastoparans, a new class of mast cell degranulating peptides from wasp venom. J Biol Chem. 1983 Nov 25;258(22):13697–13702. [PubMed] [Google Scholar]
- Bertics P. J., Chen W. S., Hubler L., Lazar C. S., Rosenfeld M. G., Gill G. N. Alteration of epidermal growth factor receptor activity by mutation of its primary carboxyl-terminal site of tyrosine self-phosphorylation. J Biol Chem. 1988 Mar 15;263(8):3610–3617. [PubMed] [Google Scholar]
- Bertics P. J., Gill G. N. Self-phosphorylation enhances the protein-tyrosine kinase activity of the epidermal growth factor receptor. J Biol Chem. 1985 Nov 25;260(27):14642–14647. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 and phospholipase C activities of platelets. Differential substrate specificity, Ca2+ requirement, pH dependence, and cellular localization. J Biol Chem. 1980 Nov 10;255(21):10227–10231. [PubMed] [Google Scholar]
- Blay J., Valentine-Braun K. A., Northup J. K., Hollenberg M. D. Epidermal-growth-factor-stimulated phosphorylation of calpactin II in membrane vesicles shed from cultured A-431 cells. Biochem J. 1989 Apr 15;259(2):577–583. doi: 10.1042/bj2590577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre J. V., Gronich J. H., Nemenoff R. A. Epidermal growth factor enhances glomerular mesangial cell soluble phospholipase A2 activity. J Biol Chem. 1990 Mar 25;265(9):4934–4938. [PubMed] [Google Scholar]
- Bonventre J. V., Swidler M. Calcium dependency of prostaglandin E2 production in rat glomerular mesangial cells. Evidence that protein kinase C modulates the Ca2+-dependent activation of phospholipase A2. J Clin Invest. 1988 Jul;82(1):168–176. doi: 10.1172/JCI113566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burch R. M. Diacylglycerol stimulates phospholipase A2 from Swiss 3T3 fibroblasts. FEBS Lett. 1988 Jul 18;234(2):283–286. doi: 10.1016/0014-5793(88)80099-1. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey M. L., Korte K., MacDonald P. C. Epidermal growth factor stimulation of prostaglandin E2 biosynthesis in amnion cells. Induction of prostaglandin H2 synthase. J Biol Chem. 1988 Jun 5;263(16):7846–7854. [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Lund K. A., Welsh J. B., Chang C. P., Walton G. M., Der C. J., Wiley H. S., Gill G. N., Rosenfeld M. G. Functional independence of the epidermal growth factor receptor from a domain required for ligand-induced internalization and calcium regulation. Cell. 1989 Oct 6;59(1):33–43. doi: 10.1016/0092-8674(89)90867-2. [DOI] [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Poenie M., Tsien R. Y., Gill G. N., Rosenfeld M. G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. 1987 Aug 27-Sep 2Nature. 328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- Clark S., Cheng D. J., Hsuan J. J., Haley J. D., Waterfield M. D. Loss of three major auto phosphorylation sites in the EGF receptor does not block the mitogenic action of EGF. J Cell Physiol. 1988 Mar;134(3):421–428. doi: 10.1002/jcp.1041340313. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Ca2+.Calmodulin-dependent release of arachidonic acid for renal medullary prostaglandin synthesis. Evidence for involvement of phospholipases A2 and C. J Biol Chem. 1983 Apr 25;258(8):4814–4823. [PubMed] [Google Scholar]
- Crouch M. F., Lapetina E. G. No direct correlation between Ca2+ mobilization and dissociation of Gi during platelet phospholipase A2 activation. Biochem Biophys Res Commun. 1988 May 31;153(1):21–30. doi: 10.1016/s0006-291x(88)81184-7. [DOI] [PubMed] [Google Scholar]
- Dunlop M., Metz S. A. A phospholipase D-like mechanism in pancreatic islet cells: stimulation by calcium ionophore, phorbol ester and sodium fluoride. Biochem Biophys Res Commun. 1989 Sep 15;163(2):922–928. doi: 10.1016/0006-291x(89)92310-3. [DOI] [PubMed] [Google Scholar]
- Fava R. A., Cohen S. Isolation of a calcium-dependent 35-kilodalton substrate for the epidermal growth factor receptor/kinase from A-431 cells. J Biol Chem. 1984 Feb 25;259(4):2636–2645. [PubMed] [Google Scholar]
- Flower R. J., Blackwell G. J. The importance of phospholipase-A2 in prostaglandin biosynthesis. Biochem Pharmacol. 1976 Feb 1;25(3):285–291. doi: 10.1016/0006-2952(76)90216-1. [DOI] [PubMed] [Google Scholar]
- George T. P., Morash S. C., Cook H. W., Byers D. M., Palmer F. B., Spence M. W. Phosphatidylcholine biosynthesis in cultured glioma cells: evidence for channeling of intermediates. Biochim Biophys Acta. 1989 Aug 22;1004(3):283–291. doi: 10.1016/0005-2760(89)90075-1. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Halenda S. P., Banga H. S., Zavoico G. B., Lau L. F., Feinstein M. B. Synergistic release of arachidonic acid from platelets by activators of protein kinase C and Ca2+ ionophores. Evidence for the role of protein phosphorylation in the activation of phospholipase A2 and independence from the Na+/H+ exchanger. Biochemistry. 1989 Sep 5;28(18):7356–7363. doi: 10.1021/bi00444a031. [DOI] [PubMed] [Google Scholar]
- Handler J. A., Danilowicz R. M., Eling T. E. Mitogenic signaling by epidermal growth factor (EGF), but not platelet-derived growth factor, requires arachidonic acid metabolism in BALB/c 3T3 cells. Modulation of EGF-dependent c-myc expression by prostaglandins. J Biol Chem. 1990 Mar 5;265(7):3669–3673. [PubMed] [Google Scholar]
- Hepler J. R., Earp H. S., Harden T. K. Long-term phorbol ester treatment down-regulates protein kinase C and sensitizes the phosphoinositide signaling pathway to hormone and growth factor stimulation. Evidence for a role of protein kinase C in agonist-induced desensitization. J Biol Chem. 1988 Jun 5;263(16):7610–7619. [PubMed] [Google Scholar]
- Hill M. A., Larkins R. G. Alterations in distribution of cardiac output in experimental diabetes in rats. Am J Physiol. 1989 Aug;257(2 Pt 2):H571–H580. doi: 10.1152/ajpheart.1989.257.2.H571. [DOI] [PubMed] [Google Scholar]
- Hirata F. The regulation of lipomodulin, a phospholipase inhibitory protein, in rabbit neutrophils by phosphorylation. J Biol Chem. 1981 Aug 10;256(15):7730–7733. [PubMed] [Google Scholar]
- Hirata Y., Uchihashi M., Nakashima H., Fujita T., Matsukura S., Matsui K. Specific receptors for epidermal growth factor in human bone tumour cells and its effect on synthesis of prostaglandin E2 by cultured osteosarcoma cell line. Acta Endocrinol (Copenh) 1984 Sep;107(1):125–130. doi: 10.1530/acta.0.1070125. [DOI] [PubMed] [Google Scholar]
- Ho A. K., Klein D. C. Activation of alpha 1-adrenoceptors, protein kinase C, or treatment with intracellular free Ca2+ elevating agents increases pineal phospholipase A2 activity. Evidence that protein kinase C may participate in Ca2+-dependent alpha 1-adrenergic stimulation of pineal phospholipase A2 activity. J Biol Chem. 1987 Aug 25;262(24):11764–11770. [PubMed] [Google Scholar]
- Kaya H., Patton G. M., Hong S. L. Bradykinin-induced activation of phospholipase A2 is independent of the activation of polyphosphoinositide-hydrolyzing phospholipase C. J Biol Chem. 1989 Mar 25;264(9):4972–4977. [PubMed] [Google Scholar]
- Levine L., Hassid A. Epidermal growth factor stimulates prostaglandin biosynthesis by canine kidney (MDCK) cells. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1181–1187. doi: 10.1016/0006-291x(77)90980-9. [DOI] [PubMed] [Google Scholar]
- Lorenzo J. A., Quinton J., Sousa S., Raisz L. G. Effects of DNA and prostaglandin synthesis inhibitors on the stimulation of bone resorption by epidermal growth factor in fetal rat long-bone cultures. J Clin Invest. 1986 Jun;77(6):1897–1902. doi: 10.1172/JCI112517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B. L., Bonventre J. V., Kremer S. G., Kudlow J. E., Skorecki K. L. Epidermal growth factor is synergistic with phorbol esters and vasopressin in stimulating arachidonate release and prostaglandin production in renal glomerular mesangial cells. Biochem J. 1988 Jan 15;249(2):587–592. doi: 10.1042/bj2490587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B. L., Holub B. J., Troyer D. A., Skorecki K. L. Epidermal growth factor stimulates phospholipase A2 in vasopressin-treated rat glomerular mesangial cells. Biochem J. 1988 Dec 1;256(2):469–474. doi: 10.1042/bj2560469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Bellot F., Honegger A. M., Ullrich A., Schlessinger J., Zilberstein A. Tyrosine kinase activity is essential for the association of phospholipase C-gamma with the epidermal growth factor receptor. Mol Cell Biol. 1990 Feb;10(2):435–441. doi: 10.1128/mcb.10.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Kruijer W., Tilly B. C., Verlaan I., Bierman A. J., de Laat S. W. Growth factor-like action of phosphatidic acid. Nature. 1986 Sep 11;323(6084):171–173. doi: 10.1038/323171a0. [DOI] [PubMed] [Google Scholar]
- Morash S. C., Cook H. W., Spence M. W. Lysophosphatidylcholine as an intermediate in phosphatidylcholine metabolism and glycerophosphocholine synthesis in cultured cells: an evaluation of the roles of 1-acyl- and 2-acyl-lysophosphatidylcholine. Biochim Biophys Acta. 1989 Aug 8;1004(2):221–229. doi: 10.1016/0005-2760(89)90271-3. [DOI] [PubMed] [Google Scholar]
- Morash S. C., Cook H. W., Spence M. W. Phosphatidylcholine metabolism in cultured cells: catabolism via glycerophosphocholine. Biochim Biophys Acta. 1988 Jul 22;961(2):194–202. doi: 10.1016/0005-2760(88)90114-2. [DOI] [PubMed] [Google Scholar]
- Moskowitz N., Andrés A., Silva W., Shapiro L., Schook W., Puszkin S. Calcium-dependent binding of calmodulin to phospholipase A2 subunits induces enzymatic activation. Arch Biochem Biophys. 1985 Sep;241(2):413–417. doi: 10.1016/0003-9861(85)90564-8. [DOI] [PubMed] [Google Scholar]
- Muldoon L. L., Rodland K. D., Magun B. E. Transforming growth factor beta modulates epidermal growth factor-induced phosphoinositide metabolism and intracellular calcium levels. J Biol Chem. 1988 Apr 15;263(11):5030–5033. [PubMed] [Google Scholar]
- Nakashima S., Nagata K., Ueeda K., Nozawa Y. Stimulation of arachidonic acid release by guanine nucleotide in saponin-permeabilized neutrophils: evidence for involvement of GTP-binding protein in phospholipase A2 activation. Arch Biochem Biophys. 1988 Mar;261(2):375–383. doi: 10.1016/0003-9861(88)90353-0. [DOI] [PubMed] [Google Scholar]
- Nolan R. D., Danilowicz R. M., Eling T. E. Role of arachidonic acid metabolism in the mitogenic response of BALB/c 3T3 fibroblasts to epidermal growth factor. Mol Pharmacol. 1988 Jun;33(6):650–656. [PubMed] [Google Scholar]
- Parker J., Daniel L. W., Waite M. Evidence of protein kinase C involvement in phorbol diester-stimulated arachidonic acid release and prostaglandin synthesis. J Biol Chem. 1987 Apr 15;262(11):5385–5393. [PubMed] [Google Scholar]
- Pepinsky R. B., Sinclair L. K. Epidermal growth factor-dependent phosphorylation of lipocortin. Nature. 1986 May 1;321(6065):81–84. doi: 10.1038/321081a0. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schonhardt T., Ferber E. Translocation of phospholipase A2 from cytosol to membranes induced by 1-oleoyl-2-acetyl-glycerol in serum-free cultured macrophages. Biochem Biophys Res Commun. 1987 Dec 16;149(2):769–775. doi: 10.1016/0006-291x(87)90434-7. [DOI] [PubMed] [Google Scholar]
- Shier W. T. Activation of high levels of endogenous phospholipase A2 in cultured cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):195–199. doi: 10.1073/pnas.76.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivka S. R., Insel P. A. Alpha 1-adrenergic receptor-mediated phosphoinositide hydrolysis and prostaglandin E2 formation in Madin-Darby canine kidney cells. Possible parallel activation of phospholipase C and phospholipase A2. J Biol Chem. 1987 Mar 25;262(9):4200–4207. [PubMed] [Google Scholar]
- Smith K. B., Losonczy I., Sahai A., Pannerselvam M., Fehnel P., Salomon D. S. Effect of 12-O-tetradecanoylphorbol-13-acetate (TPA) on the growth inhibitory and increased phosphatidylinositol (PI) responses induced by epidermal growth factor (EGF) in A431 cells. J Cell Physiol. 1983 Oct;117(1):91–100. doi: 10.1002/jcp.1041170113. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Levine L. Epidermal growth factor stimulates prostaglandin production and bone resorption in cultured mouse calvaria. Biochem Biophys Res Commun. 1978 Dec 14;85(3):966–975. doi: 10.1016/0006-291x(78)90638-1. [DOI] [PubMed] [Google Scholar]
- Wahl M., Carpenter G. Regulation of epidermal growth factor-stimulated formation of inositol phosphates in A-431 cells by calcium and protein kinase C. J Biol Chem. 1988 Jun 5;263(16):7581–7590. [PubMed] [Google Scholar]