Abstract
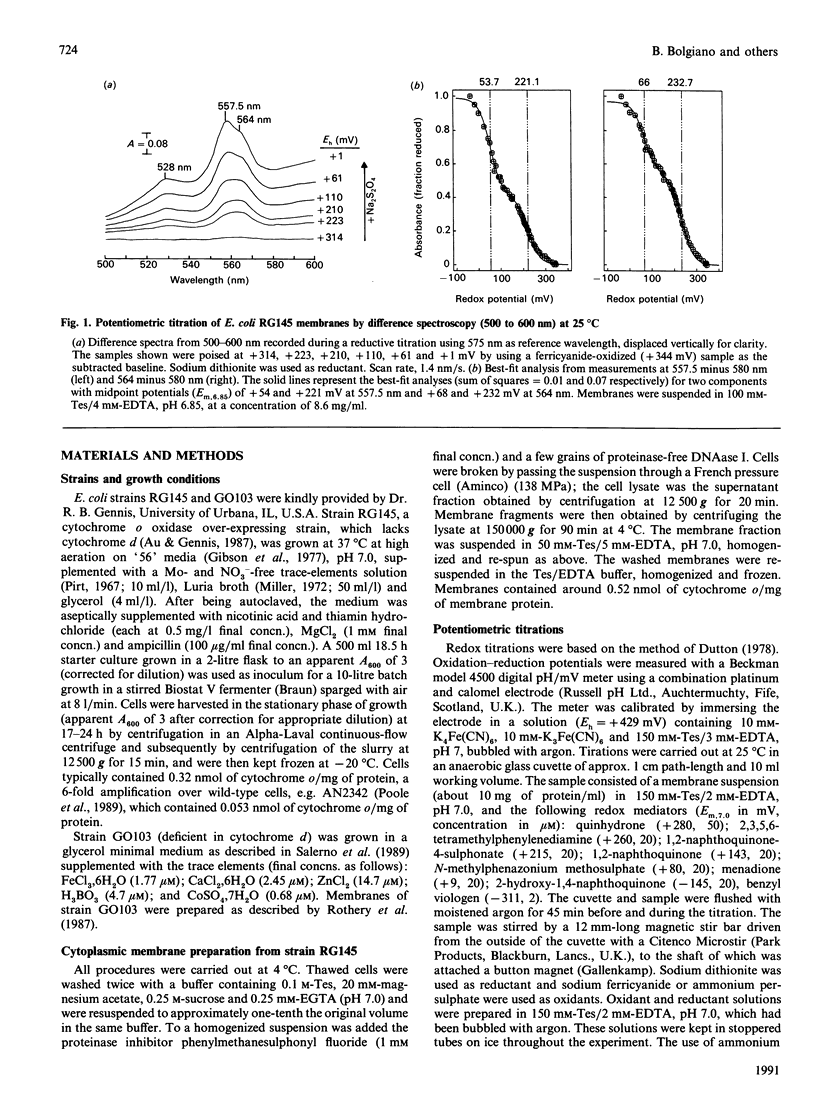
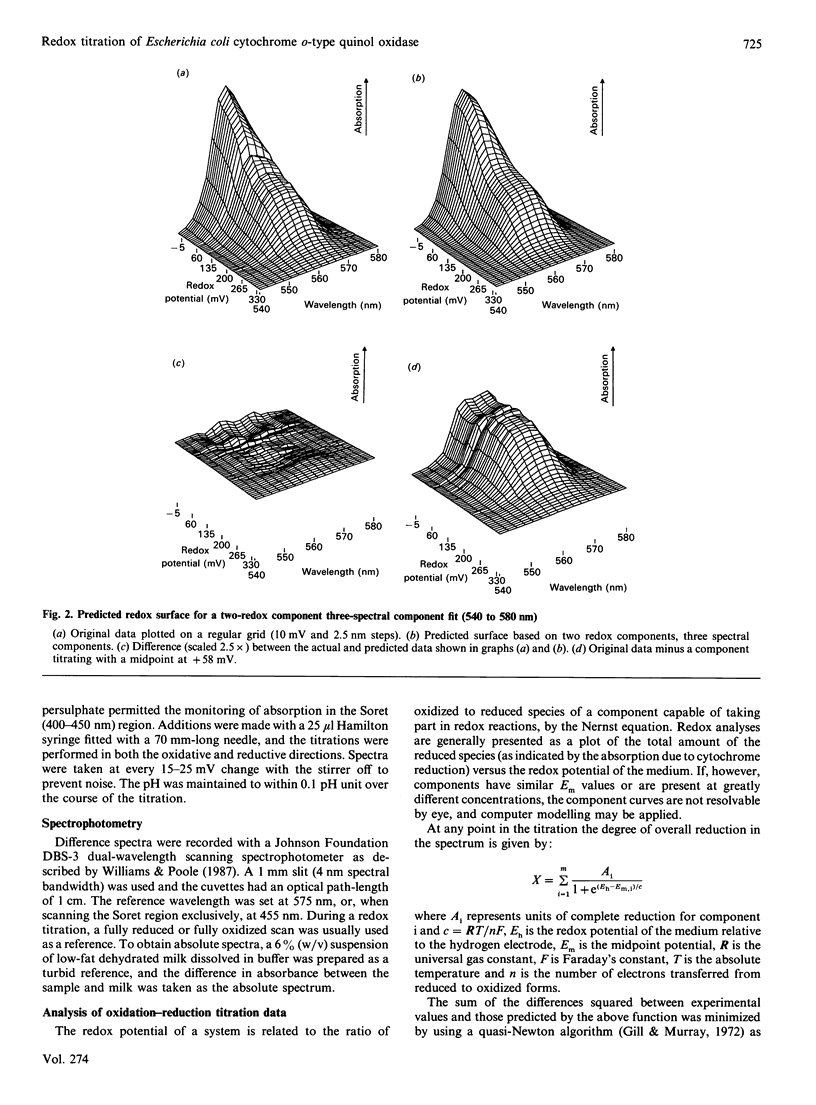
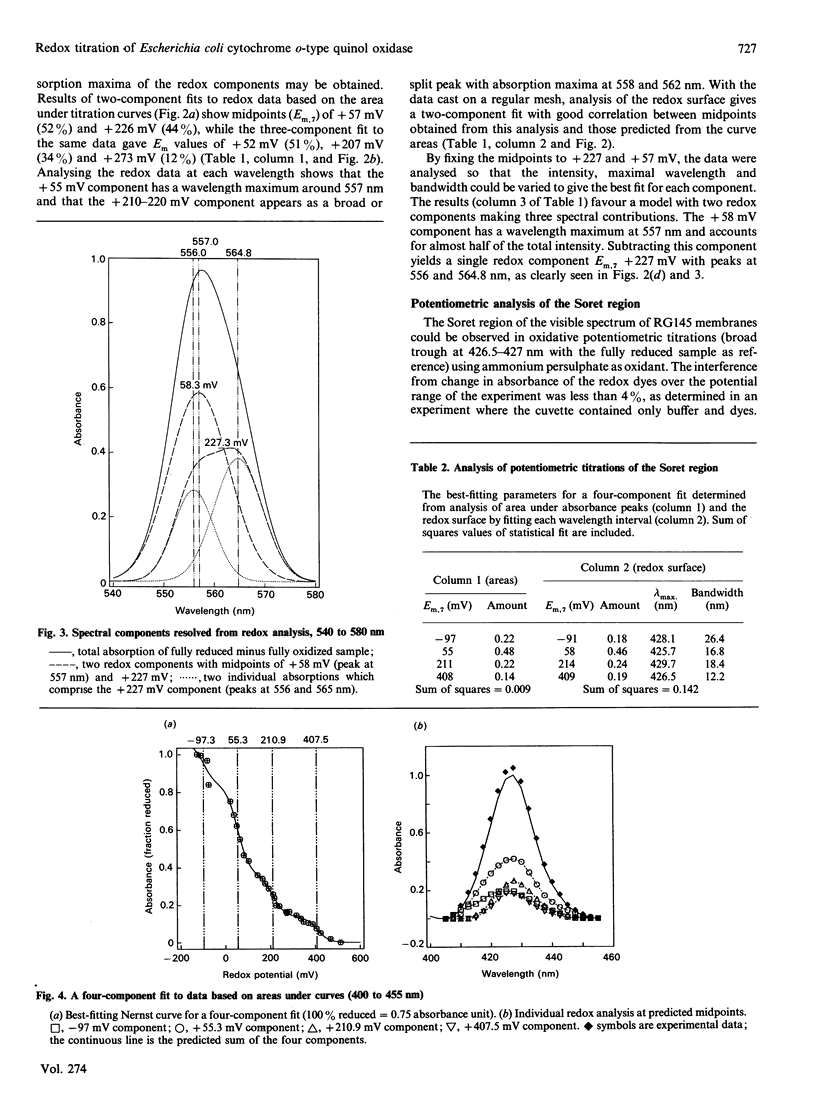
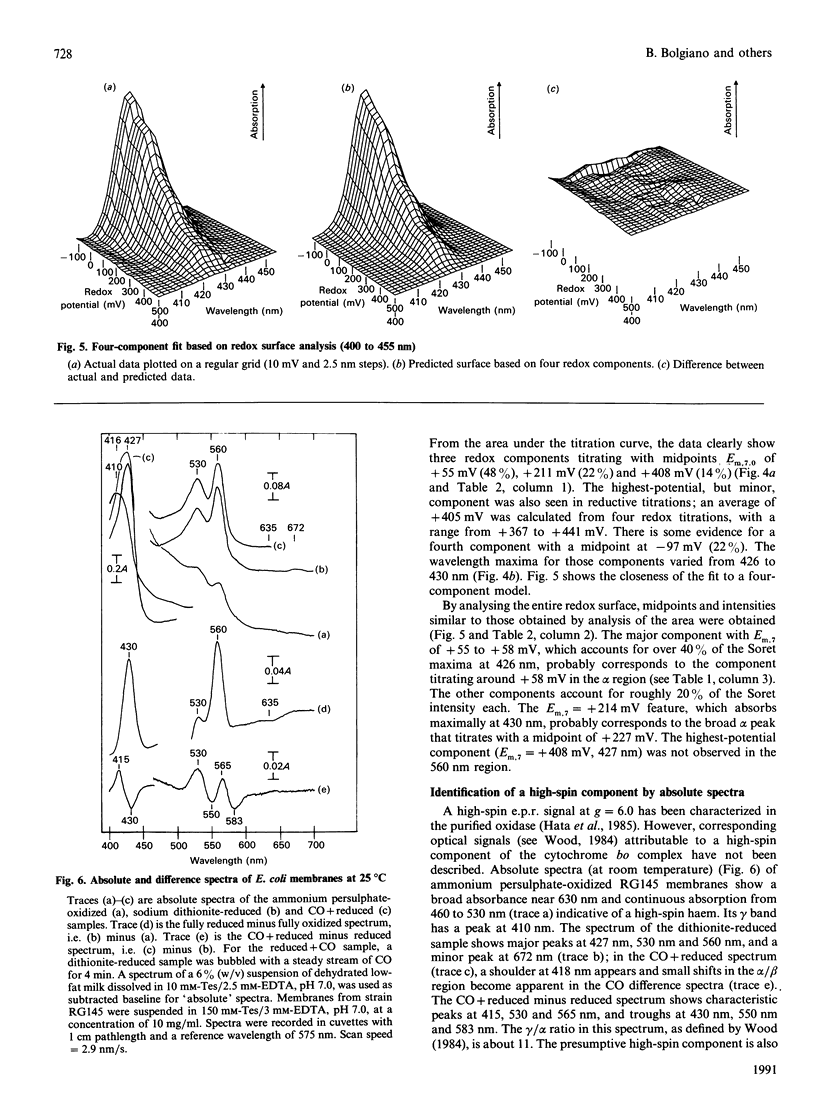
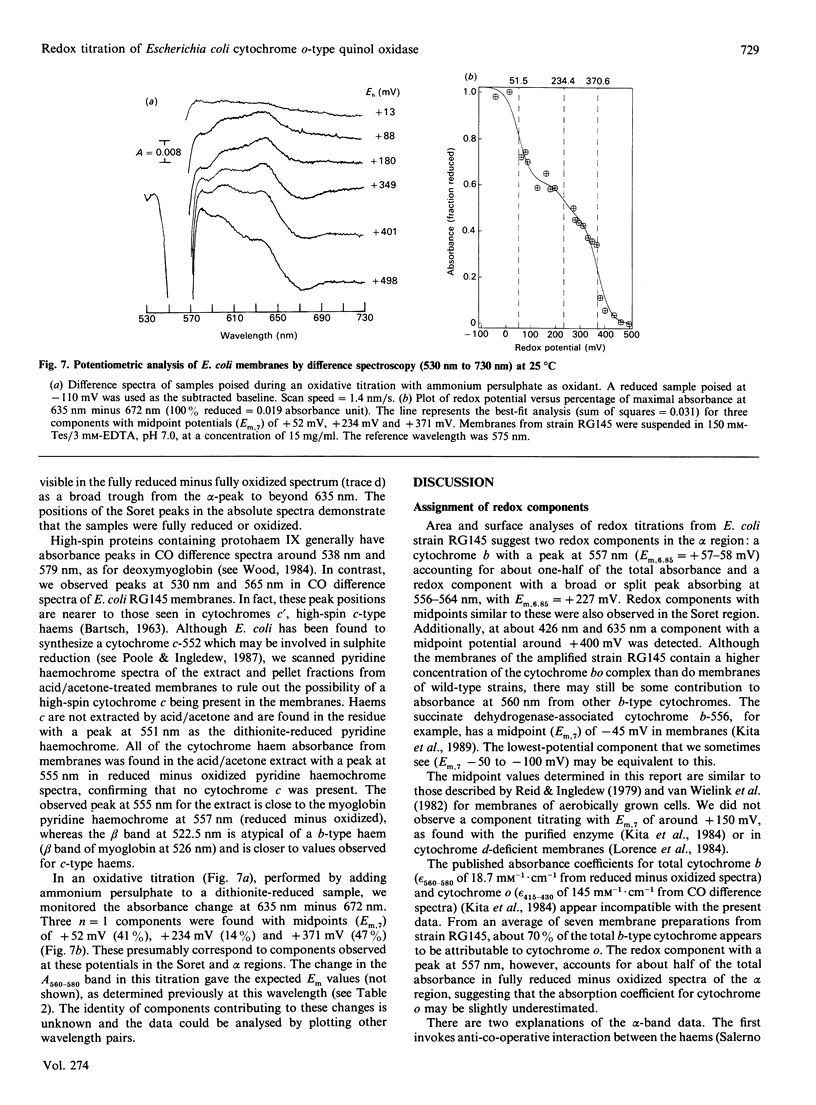
Potentiometric analyses of the cytochrome o-type oxidase of Escherichia coli, using membranes from a strain containing amplified levels of the cytochrome bo complex, were conducted to resolve the redox centres of the oxidase. The cytochrome o-type oxidase of E. coli, a quinol oxidase, contains 2 mol of b-type haem per mol of complex and copper. Detailed analysis of potentiometric titrations, based on the absorbance of the Soret band, suggests that there are three contributions with midpoint potentials (Em,7) around +55 mV, +211 mV and +408 mV, all with maxima at 426-430 nm in the reduced state. In the alpha region of the spectra, a component with Em,6.85 = +58 mV has a maximal peak at 557 nm, and twin peaks at 556 and 564 nm nitrate with Em,6.85 = +227 mV. A feature corresponding to the highest potential Soret contribution was not observed. These data can be explained either by a model incorporating haem-haem interaction or by attributing the shorter-wavelength band (557 nm) to haem b and a split alpha-band (556, 564 nm) to the haem o (oxygen-binding haem b). Absolute spectra of oxidized membranes show continuous absorbance from 460 to 530 nm and suggest the presence of a high-spin haem component in the membranes. Monitoring absorbance at 635 minus 672 nm, contributions with midpoints (Em,7) around +52 mV, +234 mV and +371 mV are observed. This latter contribution is possibly the highest-potential component which titrates with Em greater than +400 mV in the Soret region and may represent copper-haem coupling in the cytochrome o complex.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au D. C., Gennis R. B. Cloning of the cyo locus encoding the cytochrome o terminal oxidase complex of Escherichia coli. J Bacteriol. 1987 Jul;169(7):3237–3242. doi: 10.1128/jb.169.7.3237-3242.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinert H., Hansen R. E., Hartzell C. R. Kinetic studies on cytochrome c oxidase by combined epr and reflectance spectroscopy after rapid freezing. Biochim Biophys Acta. 1976 Feb 16;423(2):339–355. doi: 10.1016/0005-2728(76)90190-0. [DOI] [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- CHANCE B., SMITH L., CASTOR L. New methods for the study of the carbon monoxide compounds of respiratory enzymes. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):289–298. doi: 10.1016/0006-3002(53)90148-6. [DOI] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr, Ingledew W. J., King T. E. Low-temperature kinetics of the reaction of oxygen and solubilized cytochrome oxidase. Biochem J. 1978 Jun 1;171(3):787–798. doi: 10.1042/bj1710787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepuri V., Lemieux L., Au D. C., Gennis R. B. The sequence of the cyo operon indicates substantial structural similarities between the cytochrome o ubiquinol oxidase of Escherichia coli and the aa3-type family of cytochrome c oxidases. J Biol Chem. 1990 Jul 5;265(19):11185–11192. [PubMed] [Google Scholar]
- Dutton P. L. Redox potentiometry: determination of midpoint potentials of oxidation-reduction components of biological electron-transfer systems. Methods Enzymol. 1978;54:411–435. doi: 10.1016/s0076-6879(78)54026-3. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Chance B. Studies on the electron transport chain at subzero temperatures: electron transport at site 3. Arch Biochem Biophys. 1972 Jul;151(1):304–315. doi: 10.1016/0003-9861(72)90501-2. [DOI] [PubMed] [Google Scholar]
- Georgiou C. D., Dueweke T. J., Gennis R. B. Regulation of expression of the cytochrome d terminal oxidase in Escherichia coli is transcriptional. J Bacteriol. 1988 Feb;170(2):961–966. doi: 10.1128/jb.170.2.961-966.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Cox G. B., Downie J. A., Radik J. A mutation affecting a second component of the F0 portion of the magnesium ion-stimulated adenosine triphosphatase of Escherichia coli K12. The uncC424 allele. Biochem J. 1977 Apr 15;164(1):193–198. doi: 10.1042/bj1640193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Kirino Y., Matsuura K., Itoh S., Hiyama T., Konishi K., Kita K., Anraku Y. Assignment of ESR signals of Escherichia coli terminal oxidase complexes. Biochim Biophys Acta. 1985 Oct 29;810(1):62–72. doi: 10.1016/0005-2728(85)90206-3. [DOI] [PubMed] [Google Scholar]
- Holm L., Saraste M., Wikström M. Structural models of the redox centres in cytochrome oxidase. EMBO J. 1987 Sep;6(9):2819–2823. doi: 10.1002/j.1460-2075.1987.tb02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson B., Andréasson L. E. The identity of a new copper(II) electron paramagnetic resonance signal in cytochrome c oxidase. Biochim Biophys Acta. 1981 Mar 12;635(1):73–80. doi: 10.1016/0005-2728(81)90008-6. [DOI] [PubMed] [Google Scholar]
- Kita K., Konishi K., Anraku Y. Terminal oxidases of Escherichia coli aerobic respiratory chain. I. Purification and properties of cytochrome b562-o complex from cells in the early exponential phase of aerobic growth. J Biol Chem. 1984 Mar 10;259(5):3368–3374. [PubMed] [Google Scholar]
- Kita K., Vibat C. R., Meinhardt S., Guest J. R., Gennis R. B. One-step purification from Escherichia coli of complex II (succinate: ubiquinone oxidoreductase) associated with succinate-reducible cytochrome b556. J Biol Chem. 1989 Feb 15;264(5):2672–2677. [PubMed] [Google Scholar]
- Kranz R. G., Gennis R. B. Immunological investigation of the distribution of cytochromes related to the two terminal oxidases of Escherichia coli in other gram-negative bacteria. J Bacteriol. 1985 Feb;161(2):709–713. doi: 10.1128/jb.161.2.709-713.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. S., Jr, Wilson D. F., Owen C. S., King T. E. Heme-heme interaction in cytochrome c oxidase: the cooperativity of the hemes of cytochrome c oxidase as evidenced in the reaction with CO. Arch Biochem Biophys. 1974 Feb;160(2):476–486. doi: 10.1016/0003-9861(74)90424-x. [DOI] [PubMed] [Google Scholar]
- Lorence R. M., Green G. N., Gennis R. B. Potentiometric analysis of the cytochromes of an Escherichia coli mutant strain lacking the cytochrome d terminal oxidase complex. J Bacteriol. 1984 Jan;157(1):115–121. doi: 10.1128/jb.157.1.115-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Patel L., Kaback H. R. Cytochrome o type oxidase from Escherichia coli. Characterization of the enzyme and mechanism of electrochemical proton gradient generation. Biochemistry. 1984 Sep 25;23(20):4703–4714. doi: 10.1021/bi00315a028. [DOI] [PubMed] [Google Scholar]
- Müller M., Schläpfer B., Azzi A. Cytochrome c oxidase from Paracoccus denitrificans: both hemes are located in subunit I. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6647–6651. doi: 10.1073/pnas.85.18.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirt S. J. A kinetic study of the mode of growth of surface colonies of bacteria and fungi. J Gen Microbiol. 1967 May;47(2):181–197. doi: 10.1099/00221287-47-2-181. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Chance B. The reaction of cytochrome o in Escherichia coli K12 with oxygen. Evidence for a spectrally and kinetically distinct cytochrome o in cells from oxygen-limited cultures. J Gen Microbiol. 1981 Oct;126(2):277–287. doi: 10.1099/00221287-126-2-277. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Waring A. J., Chance B. The reaction of cytochrome omicron in Escherichia coli with oxygen. Low-temperature kinetic and spectral studies. Biochem J. 1979 Nov 15;184(2):379–389. doi: 10.1042/bj1840379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K., Williams H. D., Downie J. A., Gibson F. Mutations affecting the cytochrome d-containing oxidase complex of Escherichia coli K12: identification and mapping of a fourth locus, cydD. J Gen Microbiol. 1989 Jul;135(7):1865–1874. doi: 10.1099/00221287-135-7-1865. [DOI] [PubMed] [Google Scholar]
- Reid G. A., Ingledew W. J. Characterization and phenotypic control of the cytochrome content of Escherichia coli. Biochem J. 1979 Aug 15;182(2):465–472. doi: 10.1042/bj1820465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothery R. A., Houston A. M., Ingledew W. J. The respiratory chain of anaerobically grown Escherichia coli: reactions with nitrite and oxygen. J Gen Microbiol. 1987 Nov;133(11):3247–3255. doi: 10.1099/00221287-133-11-3247. [DOI] [PubMed] [Google Scholar]
- Salerno J. C., Bolgiano B., Ingledew W. J. Potentiometric titration of cytochrome-bo type quinol oxidase of Escherichia coli: evidence for heme-heme and copper-heme interaction. FEBS Lett. 1989 Apr 10;247(1):101–105. doi: 10.1016/0014-5793(89)81249-9. [DOI] [PubMed] [Google Scholar]
- Salerno J. C., Bolgiano B., Poole R. K., Gennis R. B., Ingledew W. J. Heme-copper and heme-heme interactions in the cytochrome bo-containing quinol oxidase of Escherichia coli. J Biol Chem. 1990 Mar 15;265(8):4364–4368. [PubMed] [Google Scholar]
- Van Wielink J. E., Oltmann L. F., Leeuwerik F. J., De Hollander J. A., Stouthamer A. H. A method for in situ characterization of b- and c-type cytochromes in Escherichia coli and in complex III from beef heart mitochondria by combined spectrum deconvolution and potentiometric analysis. Biochim Biophys Acta. 1982 Aug 20;681(2):177–190. doi: 10.1016/0005-2728(82)90021-4. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Leigh J. S., Jr Heme-heme interaction in cytochrome c oxidase in situ as measured by EPR spectroscopy. Arch Biochem Biophys. 1972 May;150(1):154–163. doi: 10.1016/0003-9861(72)90022-7. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Bacterial proteins with CO-binding b- or c-type haem. Functions and absorption spectroscopy. Biochim Biophys Acta. 1984 Dec 17;768(3-4):293–317. doi: 10.1016/0304-4173(84)90020-x. [DOI] [PubMed] [Google Scholar]


