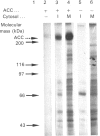
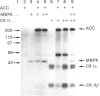
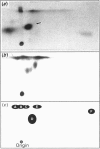
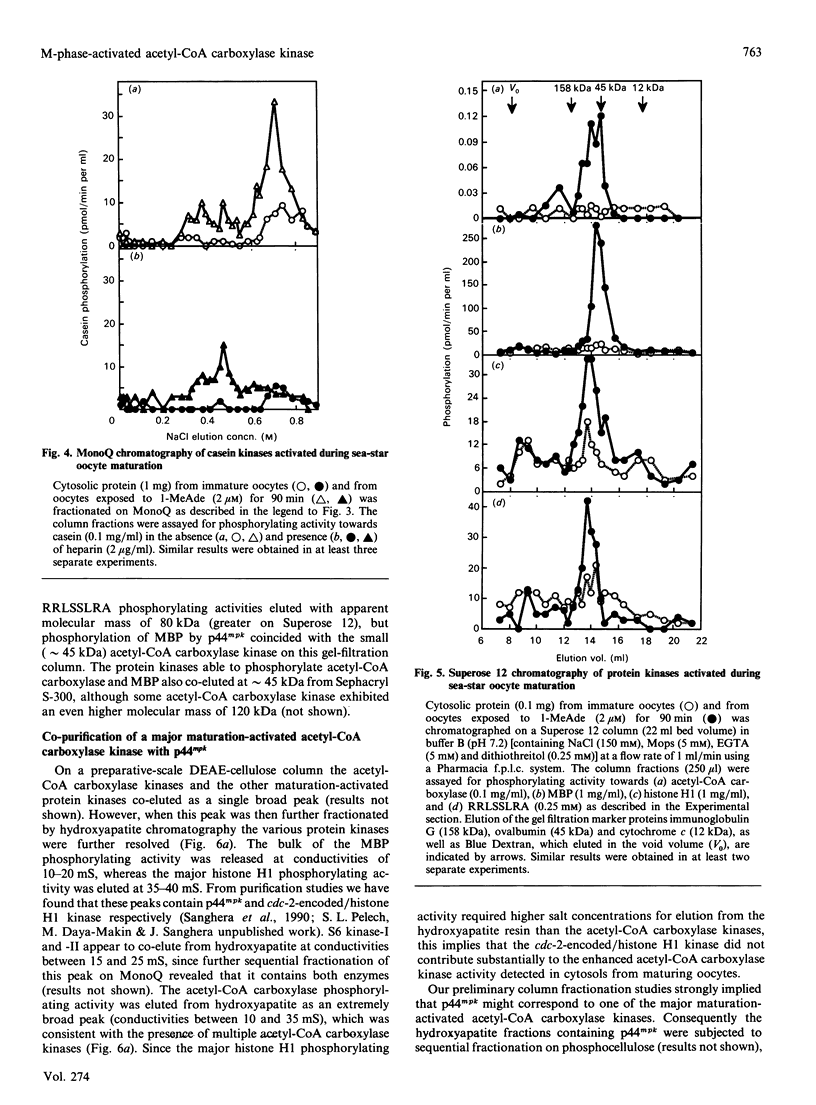
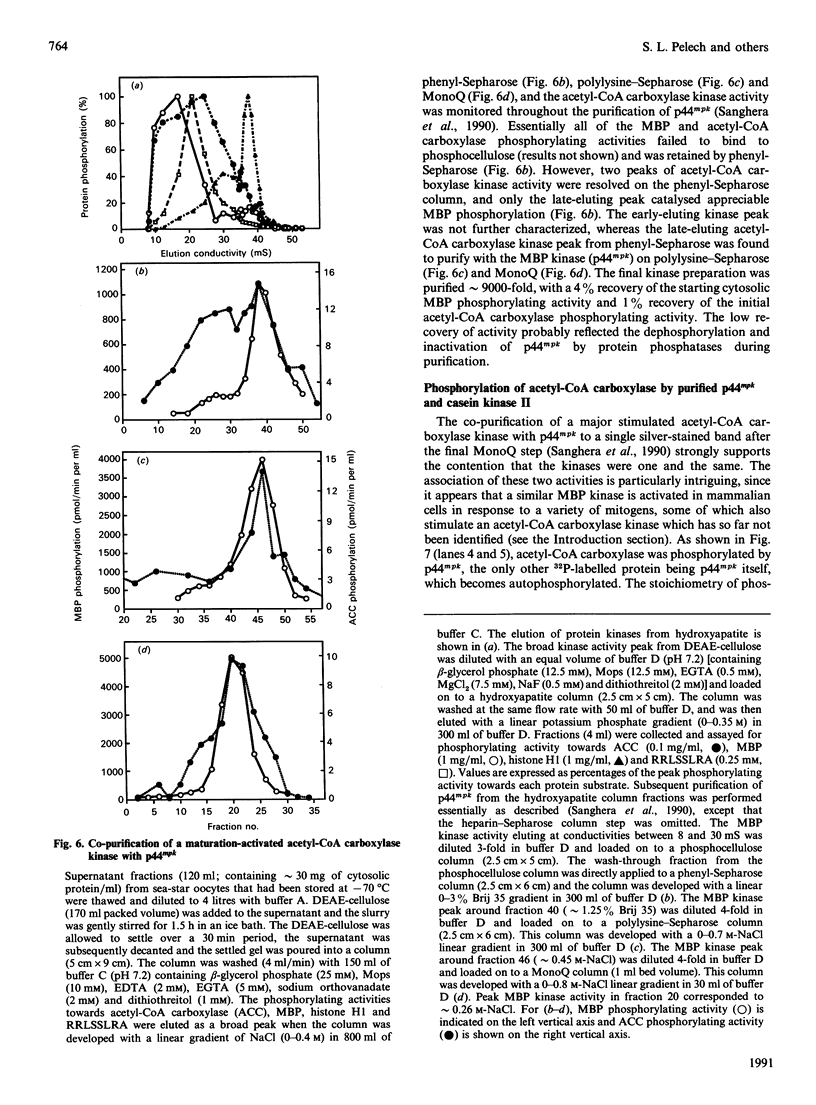
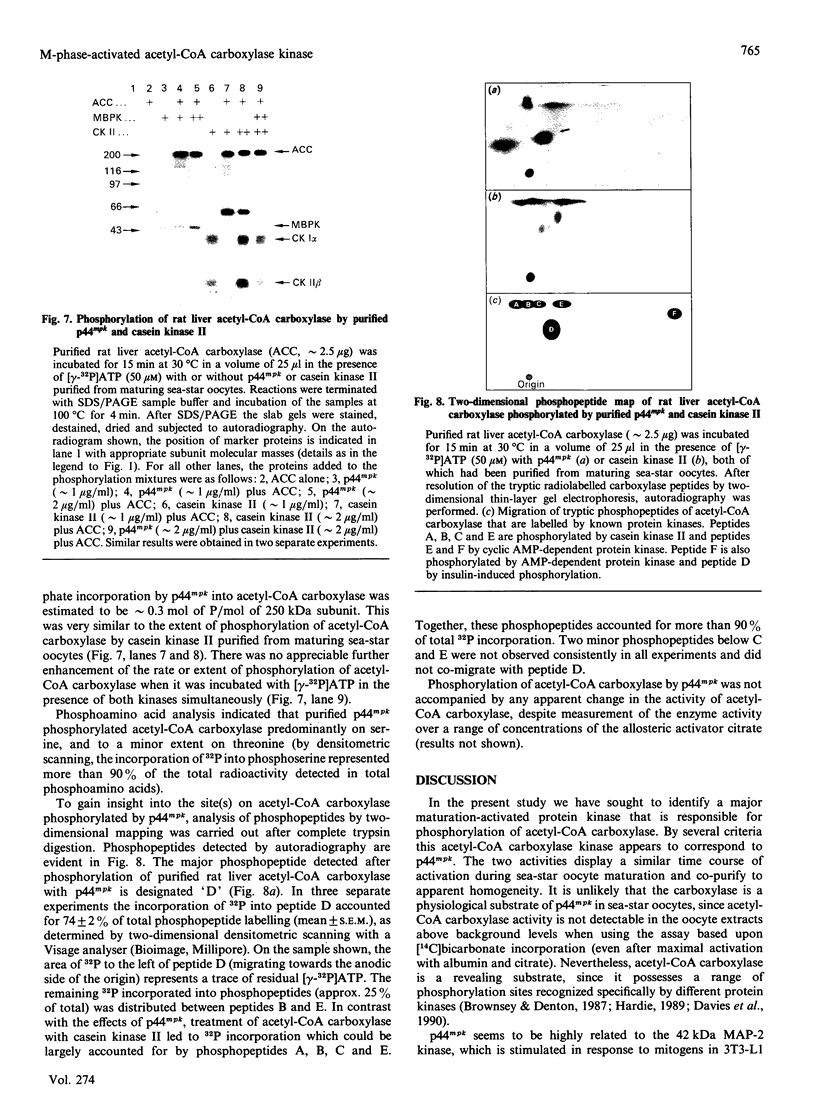
Abstract
Maturation-activated protein-serine/threonine kinases were investigated in the high-speed supernatant fractions from sea-star oocytes harvested at the time of germinal vesicle breakdown. One of the major stimulated protein kinases able to phosphorylate acetyl-CoA carboxylase in these extracts was found to co-purify with a 44 kDa myelin basic protein kinase (p44mpk) that is activated with a similar time course during oocyte maturation. Purified sea-star oocyte p44mpk phosphorylated acetyl-CoA carboxylase (purified from rat liver) predominantly on serine and to a small extent on threonine. Furthermore, the phosphorylation of acetyl-CoA carboxylase occurred principally on a tryptic phosphopeptide which displayed electrophoretic and chromatographic properties very similar to those of the peptide that has previously been shown to undergo increased phosphorylation in response to insulin in rat adipocytes [Brownsey & Denton (1982) Biochem. J. 202, 77-86]. The acetyl-CoA carboxylase was phosphorylated at a similar rate and to a similar extent by casein kinase II, which was also purified from maturing sea-star oocytes. Although casein kinase II was also activated approximately 3-fold near the time of nuclear envelope breakdown, it was responsible for only a minor component of the total enhanced acetyl-CoA carboxylase kinase activity measured in the soluble extracts from maturing oocytes. Acetyl-CoA carboxylase was a relatively poor substrate for the major S6 peptide kinase activity that was also stimulated during resumption of meiosis in the oocytes. The properties of the p44mpk are reminiscent of those of a microtubule-associated protein 2 (MAP-2) kinase that is activated in response to insulin and other mitogens in mammalian cells [Ray & Sturgill (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 3753-3757; Hoshi, Nishida & Sakai (1988) J. Biol. Chem. 263, 5396-5401]. It is intriguing that several of the mammalian protein kinases that are acutely activated after mitogenic prompting of quiescent mouse fibroblasts (i.e. G0 to G1 transition), such as MAP-2 kinase, casein kinase II and S6 kinase II, have counterparts that are activated during M-phase in maturing sea star oocytes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman P., Osheroff N. Regulation of casein kinase II activity by epidermal growth factor in human A-431 carcinoma cells. J Biol Chem. 1989 Jul 15;264(20):11958–11965. [PubMed] [Google Scholar]
- Alexander M. C., Kowaloff E. M., Witters L. A., Dennihy D. T., Avruch J. Purification of a hepatic 123,000-dalton hormone-stimulated 32P-peptide and its identification as ATP-citrate lyase. J Biol Chem. 1979 Aug 25;254(16):8052–8056. [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Arion D., Meijer L., Brizuela L., Beach D. cdc2 is a component of the M phase-specific histone H1 kinase: evidence for identity with MPF. Cell. 1988 Oct 21;55(2):371–378. doi: 10.1016/0092-8674(88)90060-8. [DOI] [PubMed] [Google Scholar]
- Benjamin W. B., Singer I. Actions of insulin, epinephrine, and dibutyryl cyclic adenosine 5'-monophosphate on fat cell protein phosphorylations. Cyclic adenosine 5'-monophosphate dependent and independent mechanisms. Biochemistry. 1975 Jul 29;14(15):3301–3309. doi: 10.1021/bi00686a003. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Witters L. A., Girard P. R., Kuo J. F., Quamo S. N. Growth factor-stimulated protein phosphorylation in 3T3-L1 cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1985 Oct 25;260(24):13304–13315. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brownsey R. W., Belsham G. J., Denton R. M. Evidence for phosphorylation and activation of acetyl CoA carboxylase by a membrane-associated cyclic AMP-independent protein kinase. Relationship to the activation of acetyl CoA carboxylase by insulin. FEBS Lett. 1981 Feb 23;124(2):145–150. doi: 10.1016/0014-5793(81)80123-8. [DOI] [PubMed] [Google Scholar]
- Brownsey R. W., Denton R. M. Evidence that insulin activates fat-cell acetyl-CoA carboxylase by increased phosphorylation at a specific site. Biochem J. 1982 Jan 15;202(1):77–86. doi: 10.1042/bj2020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownsey R. W., Dong G. W., Lam V., McGreer W. Studies on protein phosphorylation using subcellular fractions from insulin-treated white adipose tissue of rats. Biochem Cell Biol. 1988 Apr;66(4):296–308. doi: 10.1139/o88-039. [DOI] [PubMed] [Google Scholar]
- Brownsey R. W., Edgell N. J., Hopkirk T. J., Denton R. M. Studies on insulin-stimulated phosphorylation of acetyl-CoA carboxylase, ATP citrate lyase and other proteins in rat epididymal adipose tissue. Evidence for activation of a cyclic AMP-independent protein kinase. Biochem J. 1984 Mar 15;218(3):733–743. doi: 10.1042/bj2180733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D., Marshak D. R. Serum-stimulated cell growth causes oscillations in casein kinase II activity. J Biol Chem. 1989 May 5;264(13):7345–7348. [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Cicirelli M. F., Pelech S. L., Krebs E. G. Activation of multiple protein kinases during the burst in protein phosphorylation that precedes the first meiotic cell division in Xenopus oocytes. J Biol Chem. 1988 Feb 5;263(4):2009–2019. [PubMed] [Google Scholar]
- Cicirelli M. F., Pelech S. L., Krebs E. G. Insulin and progesterone activate a common synthetic ribosomal protein S6 peptide kinase in Xenopus oocytes. FEBS Lett. 1988 Dec 5;241(1-2):195–201. doi: 10.1016/0014-5793(88)81060-3. [DOI] [PubMed] [Google Scholar]
- Davies S. P., Sim A. T., Hardie D. G. Location and function of three sites phosphorylated on rat acetyl-CoA carboxylase by the AMP-activated protein kinase. Eur J Biochem. 1990 Jan 12;187(1):183–190. doi: 10.1111/j.1432-1033.1990.tb15293.x. [DOI] [PubMed] [Google Scholar]
- Deibler G. E., Boyd L. F., Kies M. W. Proteolytic activity associated with purified myelin basic protein. Prog Clin Biol Res. 1984;146:249–256. [PubMed] [Google Scholar]
- Erikson E., Maller J. L. Purification and characterization of a protein kinase from Xenopus eggs highly specific for ribosomal protein S6. J Biol Chem. 1986 Jan 5;261(1):350–355. [PubMed] [Google Scholar]
- Halestrap A. P., Denton R. M. Insulin and the regulation of adipose tissue acetyl-coenzyme A carboxylase. Biochem J. 1973 Mar;132(3):509–517. doi: 10.1042/bj1320509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie D. G. Regulation of fatty acid synthesis via phosphorylation of acetyl-CoA carboxylase. Prog Lipid Res. 1989;28(2):117–146. doi: 10.1016/0163-7827(89)90010-6. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Campbell D. G., Hardie D. G. Analysis of sites phosphorylated on acetyl-CoA carboxylase in response to insulin in isolated adipocytes. Comparison with sites phosphorylated by casein kinase-2 and the calmodulin-dependent multiprotein kinase. Eur J Biochem. 1988 Aug 1;175(2):347–354. doi: 10.1111/j.1432-1033.1988.tb14203.x. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Hardie D. G. Both insulin and epidermal growth factor stimulate lipogenesis and acetyl-CoA carboxylase activity in isolated adipocytes. Importance of homogenization procedure in avoiding artefacts in acetyl-CoA carboxylase assay. Biochem J. 1986 Mar 1;234(2):279–284. doi: 10.1042/bj2340279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haystead T. A., Hardie D. G. Evidence that activation of acetyl-CoA carboxylase by insulin in adipocytes is mediated by a low-Mr effector and not by increased phosphorylation. Biochem J. 1986 Nov 15;240(1):99–106. doi: 10.1042/bj2400099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haystead T. A., Hardie D. G. Insulin and phorbol ester stimulate phosphorylation of acetyl-CoA carboxylase at similar sites in isolated adipocytes. Lack of correspondence with sites phosphorylated on the purified enzyme by protein kinase C. Eur J Biochem. 1988 Aug 1;175(2):339–345. doi: 10.1111/j.1432-1033.1988.tb14202.x. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Moore F., Cohen P., Hardie D. G. Roles of the AMP-activated and cyclic-AMP-dependent protein kinases in the adrenaline-induced inactivation of acetyl-CoA carboxylase in rat adipocytes. Eur J Biochem. 1990 Jan 12;187(1):199–205. doi: 10.1111/j.1432-1033.1990.tb15295.x. [DOI] [PubMed] [Google Scholar]
- Hecht L. B., Straus D. S. Insulin-stimulated protein kinase activity in rat skeletal muscle that phosphorylates ribosomal protein S6. Biochem Biophys Res Commun. 1988 May 16;152(3):1200–1206. doi: 10.1016/s0006-291x(88)80412-1. [DOI] [PubMed] [Google Scholar]
- Holland R., Hardie D. G. Both insulin and epidermal growth factor stimulate fatty acid synthesis and increase phosphorylation of acetyl-CoA carboxylase and ATP-citrate lyase in isolated hepatocytes. FEBS Lett. 1985 Feb 25;181(2):308–312. doi: 10.1016/0014-5793(85)80282-9. [DOI] [PubMed] [Google Scholar]
- Hoshi M., Nishida E., Sakai H. Activation of a Ca2+-inhibitable protein kinase that phosphorylates microtubule-associated protein 2 in vitro by growth factors, phorbol esters, and serum in quiescent cultured human fibroblasts. J Biol Chem. 1988 Apr 15;263(11):5396–5401. [PubMed] [Google Scholar]
- Hoshi M., Nishida E., Sakai H. Characterization of a mitogen-activated, Ca2+-sensitive microtubule-associated protein-2 kinase. Eur J Biochem. 1989 Sep 15;184(2):477–486. doi: 10.1111/j.1432-1033.1989.tb15040.x. [DOI] [PubMed] [Google Scholar]
- Kandror K. V., Benumov A. O., Stepanov A. S. Casein kinase II from Rana temporaria oocytes. Intracellular localization and activity during progesterone-induced maturation. Eur J Biochem. 1989 Mar 15;180(2):441–448. doi: 10.1111/j.1432-1033.1989.tb14666.x. [DOI] [PubMed] [Google Scholar]
- Klarlund J. K., Czech M. P. Insulin-like growth factor I and insulin rapidly increase casein kinase II activity in BALB/c 3T3 fibroblasts. J Biol Chem. 1988 Nov 5;263(31):15872–15875. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Thrall T., Kim K. H. Hormonal regulation of acetyl CoA carboxylase effect of insulin and epinephrine. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1133–1140. doi: 10.1016/0006-291x(73)90810-3. [DOI] [PubMed] [Google Scholar]
- López-Casillas F., Bai D. H., Luo X. C., Kong I. S., Hermodson M. A., Kim K. H. Structure of the coding sequence and primary amino acid sequence of acetyl-coenzyme A carboxylase. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5784–5788. doi: 10.1073/pnas.85.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J. L. Xenopus oocytes and the biochemistry of cell division. Biochemistry. 1990 Apr 3;29(13):3157–3166. doi: 10.1021/bi00465a001. [DOI] [PubMed] [Google Scholar]
- Martin-Pérez J., Siegmann M., Thomas G. EGF, PGF2 alpha and insulin induce the phosphorylation of identical S6 peptides in swiss mouse 3T3 cells: effect of cAMP on early sites of phosphorylation. Cell. 1984 Feb;36(2):287–294. doi: 10.1016/0092-8674(84)90222-8. [DOI] [PubMed] [Google Scholar]
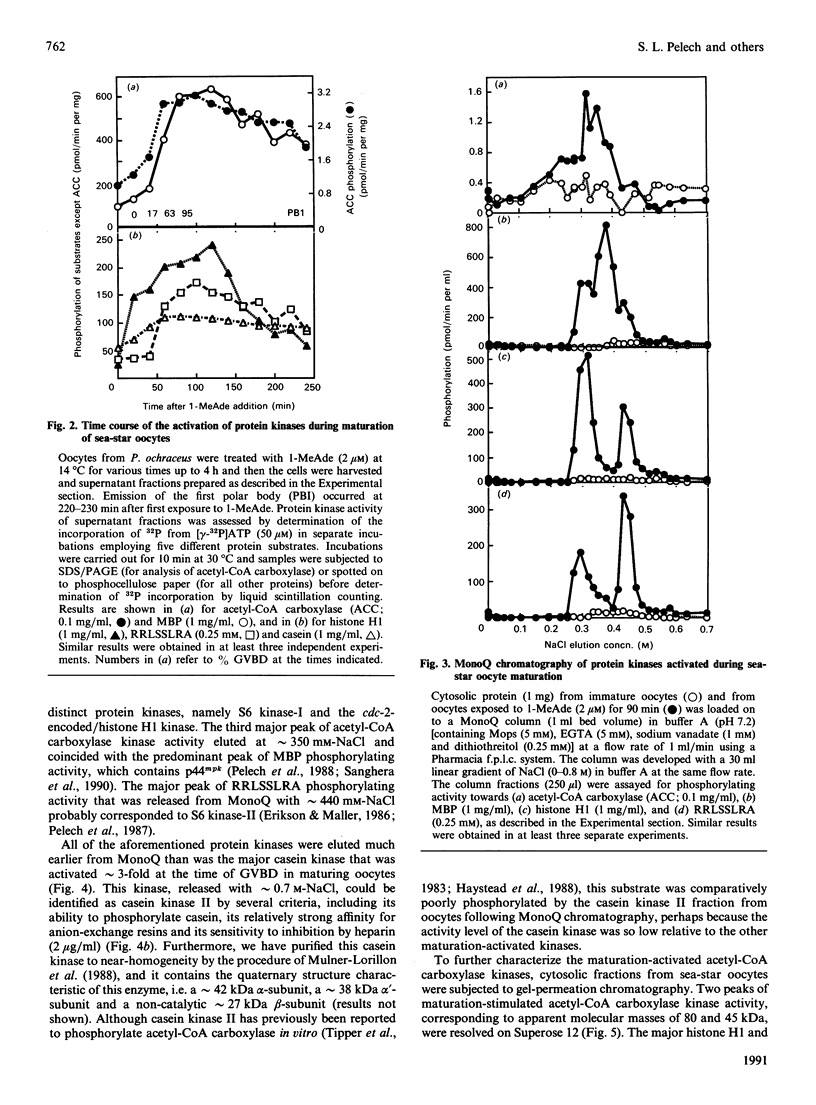
- Meijer L., Pelech S. L., Krebs E. G. Differential regulation of histone H1 and ribosomal S6 kinases during sea star oocyte maturation. Biochemistry. 1987 Dec 1;26(24):7968–7974. doi: 10.1021/bi00398a063. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Mulner-Lorillon O., Marot J., Cayla X., Pouhle R., Belle R. Purification and characterization of a casein-kinase-II-type enzyme from Xenopus laevis ovary. Biological effects on the meiotic cell division of full-grown oocyte. Eur J Biochem. 1988 Jan 15;171(1-2):107–117. doi: 10.1111/j.1432-1033.1988.tb13765.x. [DOI] [PubMed] [Google Scholar]
- Nemenoff R. A., Gunsalus J. R., Avruch J. An insulin-stimulated (ribosomal S6) protein kinase from soluble extracts of H4 hepatoma cells. Arch Biochem Biophys. 1986 Feb 15;245(1):196–203. doi: 10.1016/0003-9861(86)90205-5. [DOI] [PubMed] [Google Scholar]
- Novak-Hofer I., Thomas G. An activated S6 kinase in extracts from serum- and epidermal growth factor-stimulated Swiss 3T3 cells. J Biol Chem. 1984 May 10;259(9):5995–6000. [PubMed] [Google Scholar]
- Novak-Hofer I., Thomas G. Epidermal growth factor-mediated activation of an S6 kinase in Swiss mouse 3T3 cells. J Biol Chem. 1985 Aug 25;260(18):10314–10319. [PubMed] [Google Scholar]
- Osborne H. B., Mulner-Lorillon O., Marot J., Belle R. Polyamine levels during Xenopus laevis oogenesis: a role in oocyte competence to meiotic resumption. Biochem Biophys Res Commun. 1989 Jan 31;158(2):520–526. doi: 10.1016/s0006-291x(89)80080-4. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Krebs E. G. Mitogen-activated S6 kinase is stimulated via protein kinase C-dependent and independent pathways in Swiss 3T3 cells. J Biol Chem. 1987 Aug 25;262(24):11598–11606. [PubMed] [Google Scholar]
- Pelech S. L., Meijer L., Krebs E. G. Characterization of maturation-activated histone H1 and ribosomal S6 kinases in sea star oocytes. Biochemistry. 1987 Dec 1;26(24):7960–7968. doi: 10.1021/bi00398a062. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Olwin B. B., Krebs E. G. Fibroblast growth factor treatment of Swiss 3T3 cells activates a subunit S6 kinase that phosphorylates a synthetic peptide substrate. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5968–5972. doi: 10.1073/pnas.83.16.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S., Daya-Makin M. Protein kinase cascades in meiotic and mitotic cell cycle control. Biochem Cell Biol. 1990 Dec;68(12):1297–1330. doi: 10.1139/o90-194. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Tombes R. M., Meijer L., Krebs E. G. Activation of myelin basic protein kinases during echinoderm oocyte maturation and egg fertilization. Dev Biol. 1988 Nov;130(1):28–36. doi: 10.1016/0012-1606(88)90410-1. [DOI] [PubMed] [Google Scholar]
- Price D. J., Nemenoff R. A., Avruch J. Purification of a hepatic S6 kinase from cycloheximide-treated Rats. J Biol Chem. 1989 Aug 15;264(23):13825–13833. [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Characterization of insulin-stimulated microtubule-associated protein kinase. Rapid isolation and stabilization of a novel serine/threonine kinase from 3T3-L1 cells. J Biol Chem. 1988 Sep 5;263(25):12721–12727. [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. D., Glaccum M. B., Fischer E. H., Krebs E. G. Primary-structure requirements for inhibition by the heat-stable inhibitor of the cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1613–1616. doi: 10.1073/pnas.83.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommercorn J., Mulligan J. A., Lozeman F. J., Krebs E. G. Activation of casein kinase II in response to insulin and to epidermal growth factor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8834–8838. doi: 10.1073/pnas.84.24.8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spach D. H., Nemenoff R. A., Blackshear P. J. Protein phosphorylation and protein kinase activities in BC3H-1 myocytes. Differences between the effects of insulin and phorbol esters. J Biol Chem. 1986 Sep 25;261(27):12750–12753. [PubMed] [Google Scholar]
- Stith B. J., Maller J. L. The effect of insulin on intracellular ph and ribosomal protein S6 phosphorylation in oocytes of Xenopus laevis. Dev Biol. 1984 Mar;102(1):79–89. doi: 10.1016/0012-1606(84)90176-3. [DOI] [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Tipper J. P., Bacon G. W., Witters L. A. Phosphorylation of acetyl-coenzyme A carboxylase by casein kinase I and casein kinase II. Arch Biochem Biophys. 1983 Dec;227(2):386–396. doi: 10.1016/0003-9861(83)90468-x. [DOI] [PubMed] [Google Scholar]
- Wettenhall R. E., Morgan F. J. Phosphorylation of hepatic ribosomal protein S6 on 80 and 40 S ribosomes. Primary structure of S6 in the region of the major phosphorylation sites for cAMP-dependent protein kinases. J Biol Chem. 1984 Feb 25;259(4):2084–2091. [PubMed] [Google Scholar]
- Witters L. A. Insulin stimulates the phosphorylation of acetyl-CoA carboxylase. Biochem Biophys Res Commun. 1981 May 29;100(2):872–878. doi: 10.1016/s0006-291x(81)80254-9. [DOI] [PubMed] [Google Scholar]
- Witters L. A., Tipper J. P., Bacon G. W. Stimulation of site-specific phosphorylation of acetyl coenzyme A carboxylase by insulin and epinephrine. J Biol Chem. 1983 May 10;258(9):5643–5648. [PubMed] [Google Scholar]