Abstract
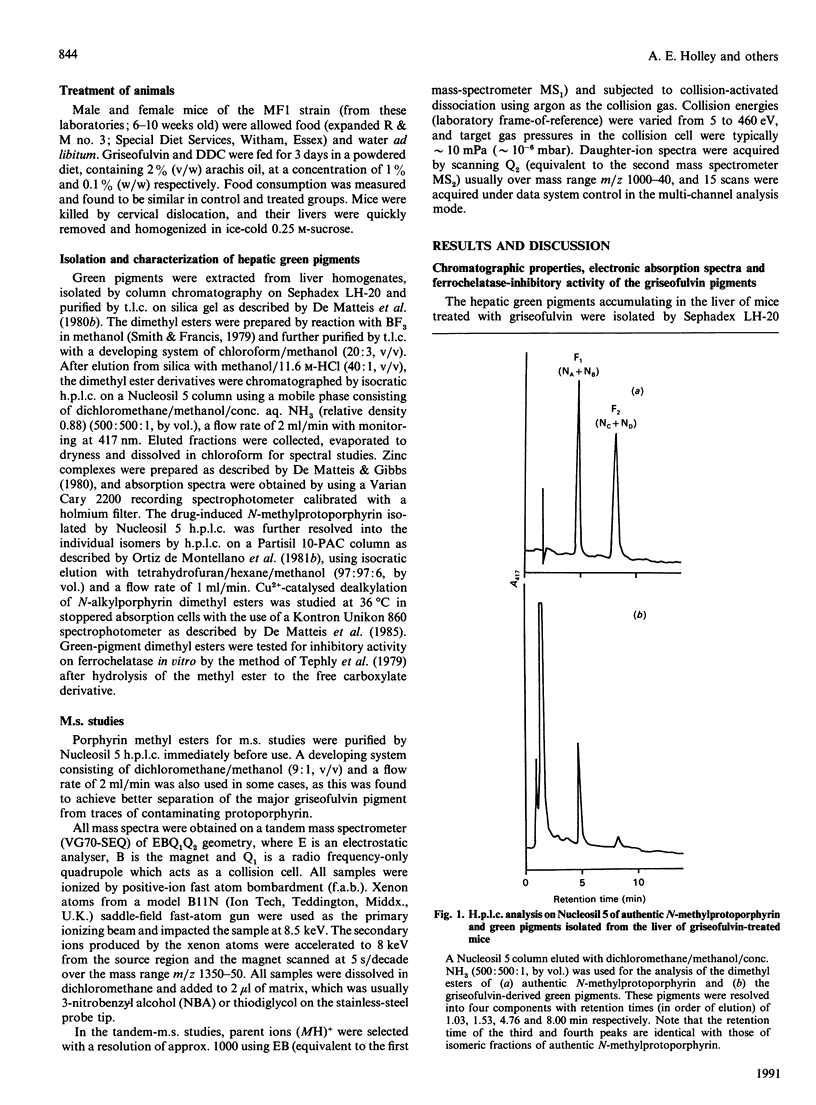
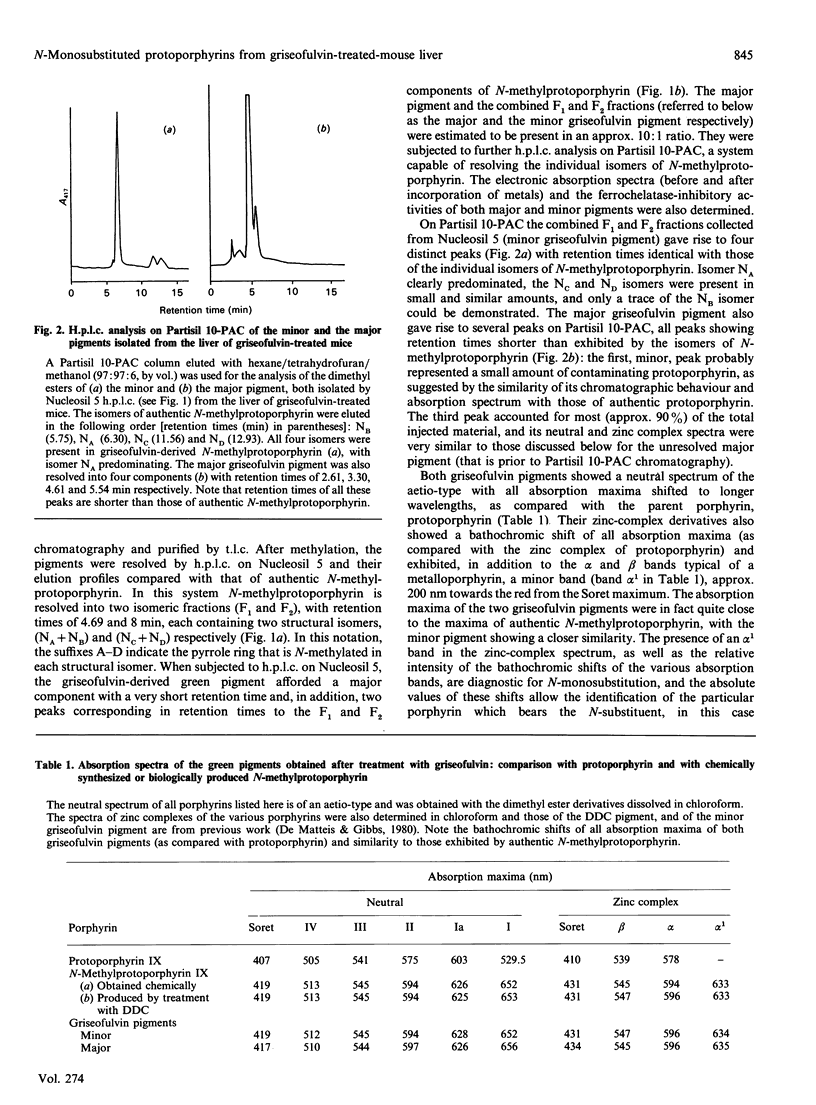
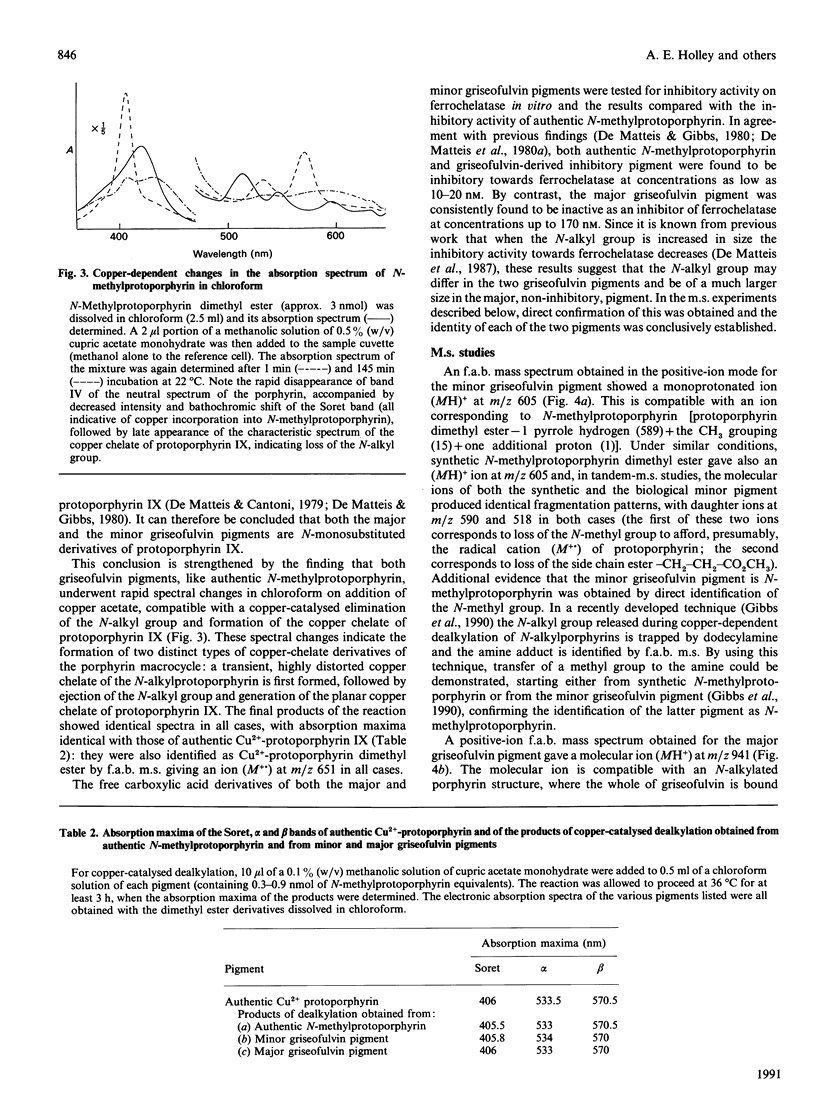
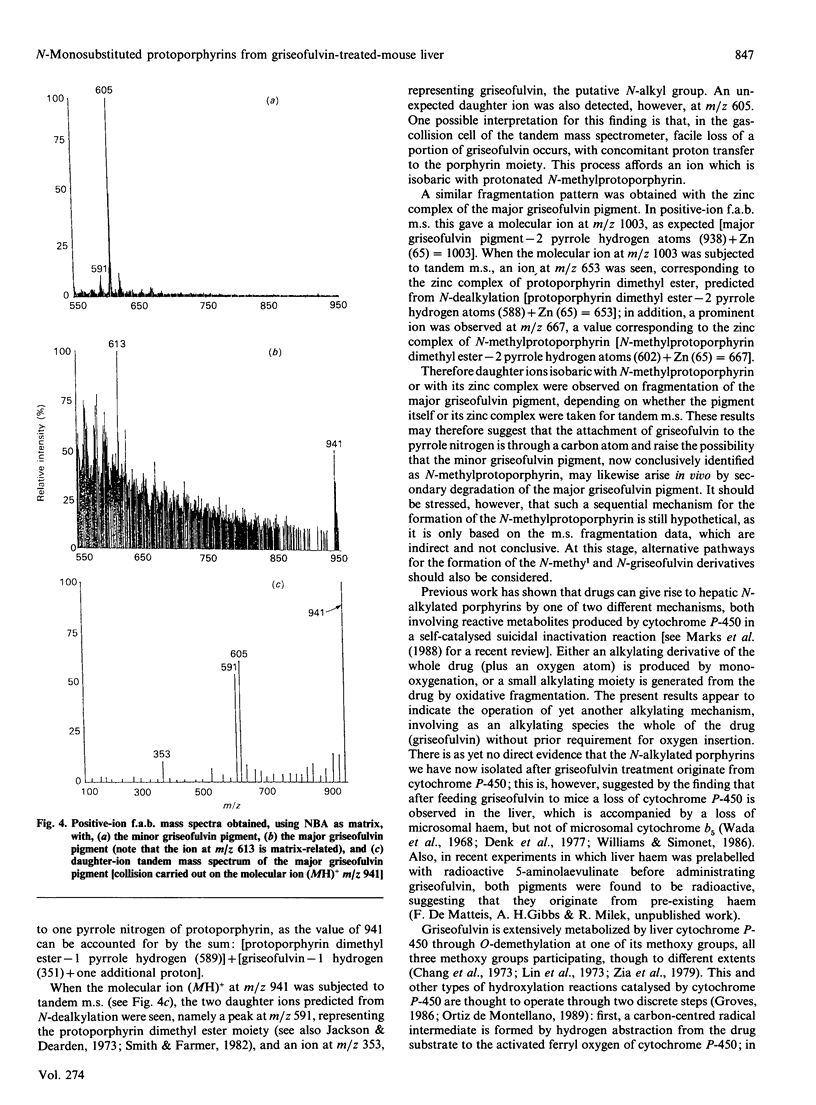
1. A hepatic green pigment with inhibitory properties towards the enzyme ferrochelatase has been isolated from the liver of mice treated with griseofulvin and identified as N-methylprotoporphyrin. 2. All four structural isomers of N-methylprotoporphyrin have been demonstrated to be present, NA, where ring A of protoporphyrin IX is N-methylated, being the predominant isomer. 3. In addition to N-methylprotoporphyrin, a second green pigment, present in far greater amounts, was also isolated from the liver of griseofulvin-treated mice. This second green pigment is also an N-monosubstituted protoporphyrin, but in this case the substituent on the pyrrole nitrogen atom appears to be intact griseofulvin rather than a methyl group. 4. The fragmentation of this adduct in tandem m.s. studies suggests that griseofulvin is bound to the pyrrole nitrogen through one of its carbon atoms and further suggests that N-methylprotoporphyrin may arise as a secondary product from the major griseofulvin pigment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang R. L., Symchowicz S., Lin C. C. Oxidative demethylation of 14C-griseofulvin by liver microsomes of rats and mice. Biochem Pharmacol. 1973 Jun 1;22(11):1389–1392. doi: 10.1016/0006-2952(73)90314-6. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Cantoni L. Alteration of the porphyrin nucleus of cytochrome P-450 caused in the liver by treatment with allyl-containing drugs. Is the modified porphyrin N-substituted? Biochem J. 1979 Oct 1;183(1):99–103. doi: 10.1042/bj1830099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H. Drug-induced conversion of liver haem into modified porphyrins. Evidence for two classes of products. Biochem J. 1980 Apr 1;187(1):285–288. doi: 10.1042/bj1870285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H., Farmer P. B., Lamb J. H. Liver production of N-alkylated porphyrins caused in mice by treatment with substituted dihydropyridines. Evidence that the alkyl group on the pyrrole nitrogen atom originates from the drug. FEBS Lett. 1981 Jul 6;129(2):328–331. doi: 10.1016/0014-5793(81)80194-9. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H., Harvey C. Studies on the inhibition of ferrochelatase by N-alkylated dicarboxylic porphyrins. Steric factors involved and evidence that the inhibition is reversible. Biochem J. 1985 Mar 1;226(2):537–544. doi: 10.1042/bj2260537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H., Holley A. E. Occurrence and biological properties of N-methyl protoporphyrin. Ann N Y Acad Sci. 1987;514:30–40. doi: 10.1111/j.1749-6632.1987.tb48758.x. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H., Tephly T. R. Inhibition of protohaem ferro-lyase in experimental porphyria. Isolation and partial characterization of a modified porphyrin inhibitor. Biochem J. 1980 Apr 15;188(1):145–152. doi: 10.1042/bj1880145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk H., Eckerstorfer R., Talcott R. E., Schenkman J. B. Alteration of hepatic microsomal enzymes by griseofulvin treatment of mice. Biochem Pharmacol. 1977 Jun 15;26(12):1125–1130. doi: 10.1016/0006-2952(77)90055-7. [DOI] [PubMed] [Google Scholar]
- Groves J. T. Biological strategies for the manipulation of dioxygen. The chemistry of cytochrome P-450. Ann N Y Acad Sci. 1986;471:99–107. doi: 10.1111/j.1749-6632.1986.tb48029.x. [DOI] [PubMed] [Google Scholar]
- Jackson A. H., Dearden G. R. N-methylporphyrins. Ann N Y Acad Sci. 1973;206:151–176. doi: 10.1111/j.1749-6632.1973.tb43210.x. [DOI] [PubMed] [Google Scholar]
- Lin C. C., Magat J., Chang R., McGlotten J., Symchowicz S. Absorption, metabolism and excretion of 14C-griseofulvin in man. J Pharmacol Exp Ther. 1973 Nov;187(2):415–422. [PubMed] [Google Scholar]
- Marks G. S., Allen D. T., Johnston C. T., Sutherland E. P., Nakatsu K., Whitney R. A. Suicidal destruction of cytochrome P-450 and reduction of ferrochelatase activity by 3,5-diethoxycarbonyl-1,4-dihydro-2,4,6-trimethylpyridine and its analogues in chick embryo liver cells. Mol Pharmacol. 1985 Apr;27(4):459–465. [PubMed] [Google Scholar]
- Marks G. S., McCluskey S. A., Mackie J. E., Riddick D. S., James C. A. Disruption of hepatic heme biosynthesis after interaction of xenobiotics with cytochrome P-450. FASEB J. 1988 Sep;2(12):2774–2783. doi: 10.1096/fasebj.2.12.3044903. [DOI] [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Beilan H. S., Kunze K. L. N-Alkylprotoporphyrin IX formation in 3,5-dicarbethoxy-1,4-dihydrocollidine-treated rats. Transfer of the alkyl group from the substrate to the porphyrin. J Biol Chem. 1981 Jul 10;256(13):6708–6713. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Beilan H. S., Kunze K. L. N-Methylprotoporphyrin IX: chemical synthesis and identification as the green pigment produced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine treatment. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1490–1494. doi: 10.1073/pnas.78.3.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz de Montellano P. R. Cytochrome P-450 catalysis: radical intermediates and dehydrogenation reactions. Trends Pharmacol Sci. 1989 Sep;10(9):354–359. doi: 10.1016/0165-6147(89)90007-2. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Francis J. E. Decarboxylation of porphyrinogens by rat liver uroporphyrinogen decarboxylase. Biochem J. 1979 Nov 1;183(2):455–458. doi: 10.1042/bj1830455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tephly T. R., Coffman B. L., Ingall G., Ziet-Har M. S., Goff H. M., Tabba H. D., Smith K. M. Identification of N-methylprotoporphyrin IX in livers of untreated mice and mice treated with 3, 5-diethoxycarbonyl- 1, 4-dihydrocollidine: source of the methyl group. Arch Biochem Biophys. 1981 Nov;212(1):120–126. doi: 10.1016/0003-9861(81)90350-7. [DOI] [PubMed] [Google Scholar]
- Tephly T. R., Gibbs A. H., De Matteis F. Studies on the mechanism of experimental porphyria produced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Role of a porphyrin-like inhibitor of protohaem ferro-lyase. Biochem J. 1979 Apr 15;180(1):241–244. doi: 10.1042/bj1800241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tephly T. R., Gibbs A. H., Ingall G., De Matteis F. Studies on the mechanism of experimental porphyria and ferrochelatase inhibition produced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Int J Biochem. 1980;12(5-6):993–998. doi: 10.1016/0020-711x(80)90200-1. [DOI] [PubMed] [Google Scholar]
- Wada O., Yano Y., Urata G., Nakao K. Behavior of hepatic microsomal cytochromes after treatment of mice with drugs known to disturb porphyrin metabolism in liver. Biochem Pharmacol. 1968 Apr;17(4):595–603. doi: 10.1016/0006-2952(68)90275-x. [DOI] [PubMed] [Google Scholar]
- Williams M. T., Simonet L. Effects of griseofulvin on enzymes associated with phase I and II of drug metabolism. Biochem Pharmacol. 1986 Aug 1;35(15):2630–2632. doi: 10.1016/0006-2952(86)90065-1. [DOI] [PubMed] [Google Scholar]
- Zia H., O'Donnell J. P., Ma J. K. Identification of griseofulvic acid as a urine metabolite of griseofulvin in humans. J Pharm Sci. 1979 Oct;68(10):1335–1336. doi: 10.1002/jps.2600681042. [DOI] [PubMed] [Google Scholar]
- de Matteis F., Gibbs A. H., Jackson A. H., Weerasinghe S. Conversion of liver haem into N-substituted porphyrins or green pigments. Nature of the substituent at the pyrrole nitrogen atom. FEBS Lett. 1980 Sep 22;119(1):109–112. doi: 10.1016/0014-5793(80)81009-x. [DOI] [PubMed] [Google Scholar]


