Abstract
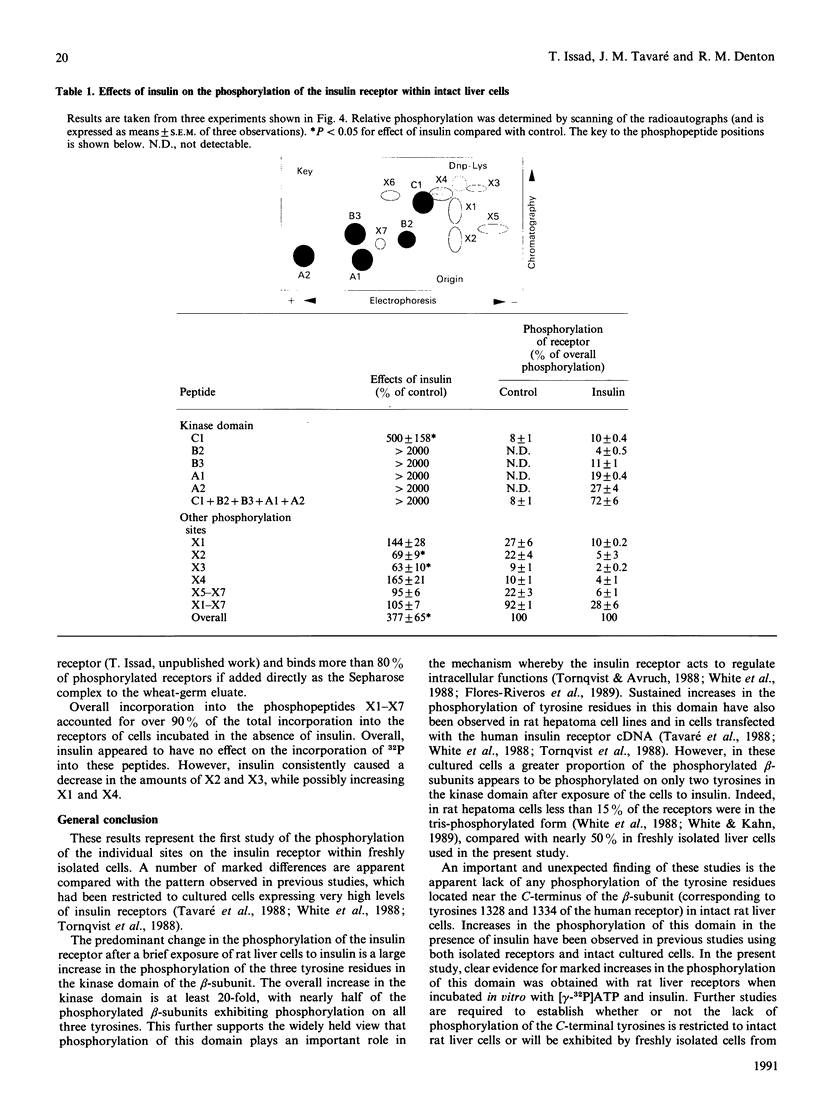
1. Insulin receptors were partially purified from rat liver by chromatography on wheat-germ-lectin-Sepharose. Incubation with [gamma-32P]ATP in the presence of insulin resulted in increased phosphorylation of the beta-subunit on both tyrosine and serine residues. Two-dimensional mapping of tryptic peptides showed that, in agreement with previous studies using preparations of receptors from other sources, the tyrosine residues involved were the three tyrosines in the kinase domain (corresponding to tyrosines 1158, 1162 and 1163 of the human receptor) plus two tyrosines close to the C-terminus (corresponding to tyrosines 1328 and 1334). 2. The effects of insulin on the phosphorylation of receptors within intact rat liver cells were determined by incubating cells in the presence of [32P]Pi for 50 min and then with or without insulin for a further 10 min. The labelled receptors were then rapidly isolated by sequential use of wheat-germ-lectin-Sepharose chromatography and immuno-isolation using a monoclonal antibody to the C-terminal end of the beta-subunit. 3. Insulin was found to increase overall phosphorylation of the receptor nearly 3-fold. Two-dimensional mapping was then carried out in combination with phosphoamino acid analysis. This revealed that the pattern of phosphorylation of the receptors in cells incubated in the absence and presence of insulin exhibited a number of marked differences from that observed in previous studies on intact cells, which had been restricted to cells expressing very high levels of insulin receptors such as certain hepatoma-derived cells or cells transfected with insulin receptor cDNA. The differences in the effects of insulin included a larger increase in the proportion of receptors being phosphorylated on the three tyrosine residues of the kinase domain, no apparent phosphorylation of the two tyrosine residues close to the C-terminus and no increase in either threonine or overall serine phosphorylation. 4. The receptors appeared to be phosphorylated on a number of different serine residues in cells incubated in the absence of insulin. Evidence for both increases and decreases in the phosphorylation of specific serine residues on addition of insulin was obtained. 5. It is concluded that care should be taken when extrapolating findings on the phosphorylation of the insulin receptor within cultured cells to more physiological situations.
Full text
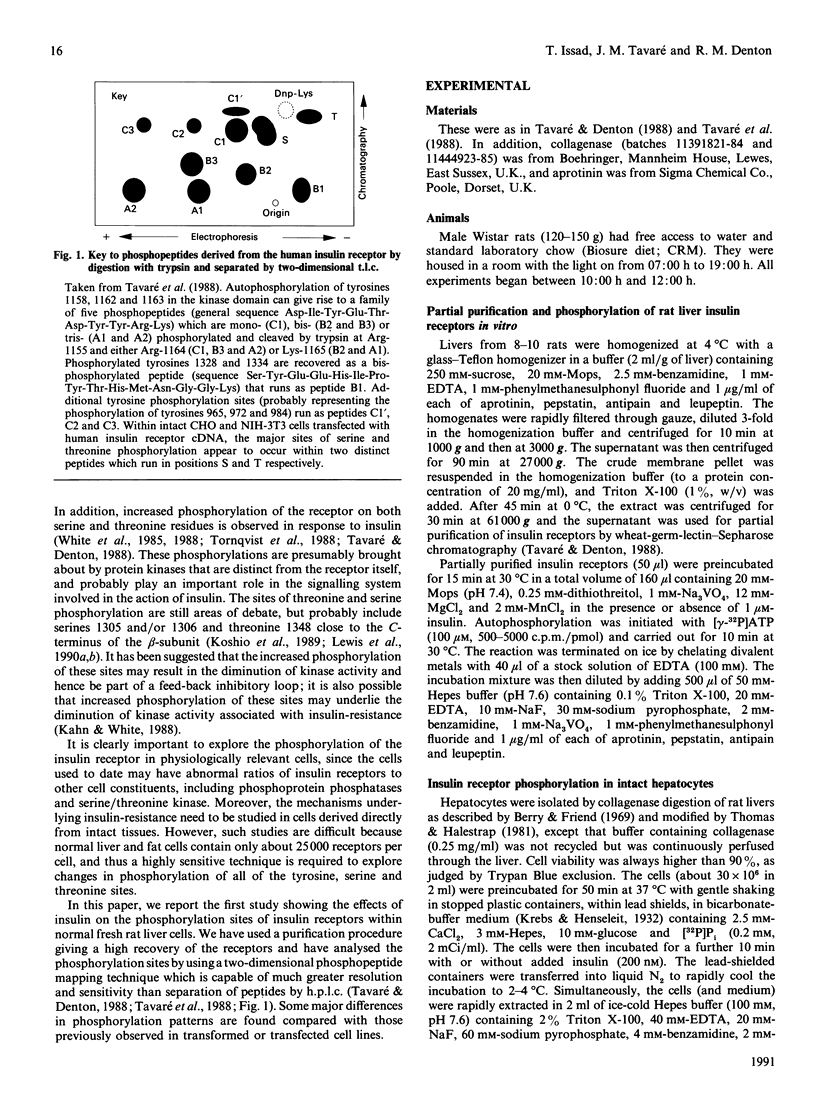
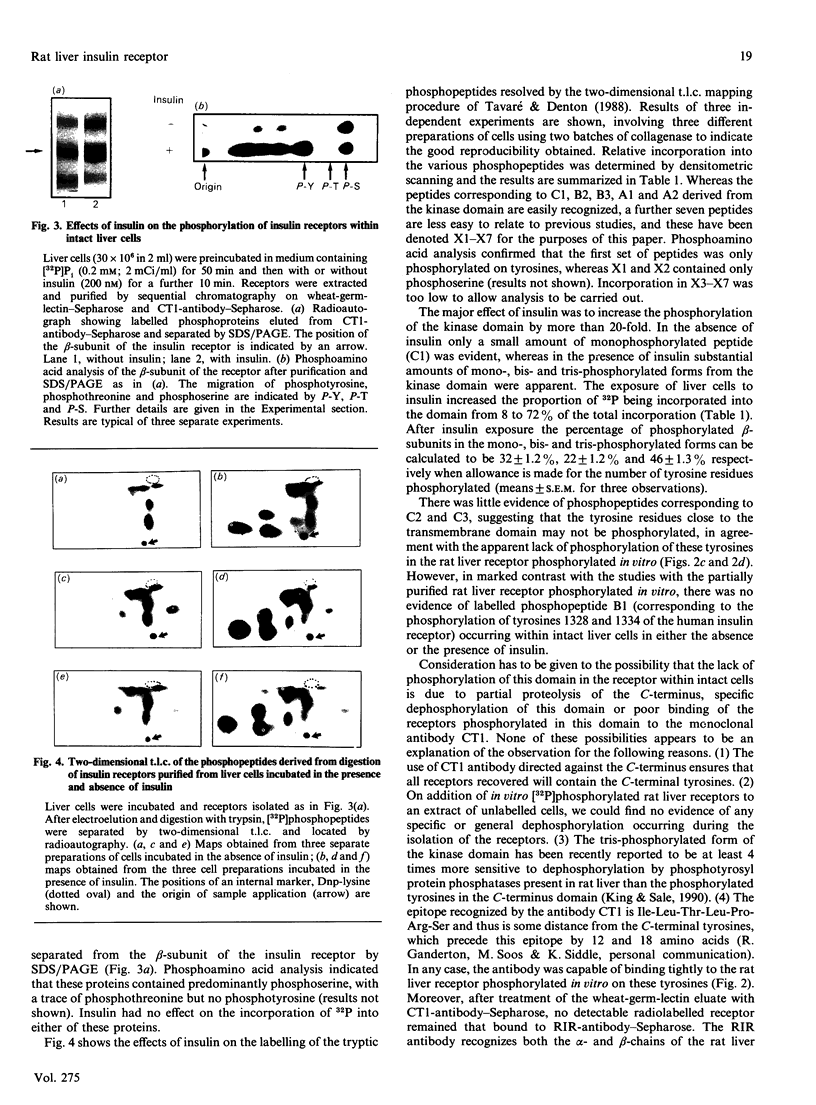
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews D. L., Beames B., Summers M. D., Park W. D. Characterization of the lipid acyl hydrolase activity of the major potato (Solanum tuberosum) tuber protein, patatin, by cloning and abundant expression in a baculovirus vector. Biochem J. 1988 May 15;252(1):199–206. doi: 10.1042/bj2520199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballotti R., Kowalski A., White M. F., Le Marchand-Brustel Y., Van Obberghen E. Insulin stimulates tyrosine phosphorylation of its receptor beta-subunit in intact rat hepatocytes. Biochem J. 1987 Jan 1;241(1):99–104. doi: 10.1042/bj2410099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro J. F., Sinha M. K., Raju S. M., Ittoop O., Pories W. J., Flickinger E. G., Meelheim D., Dohm G. L. Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. J Clin Invest. 1987 May;79(5):1330–1337. doi: 10.1172/JCI112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Denton R. M. Early events in insulin actions. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:293–341. [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Flores-Riveros J. R., Sibley E., Kastelic T., Lane M. D. Substrate phosphorylation catalyzed by the insulin receptor tyrosine kinase. Kinetic correlation to autophosphorylation of specific sites in the beta subunit. J Biol Chem. 1989 Dec 25;264(36):21557–21572. [PubMed] [Google Scholar]
- Goldstein B. J., Dudley A. L. The rat insulin receptor: primary structure and conservation of tissue-specific alternative messenger RNA splicing. Mol Endocrinol. 1990 Feb;4(2):235–244. doi: 10.1210/mend-4-2-235. [DOI] [PubMed] [Google Scholar]
- Hopkirk T. J., Denton R. M. Studies on the specific activity of [gamma-32P]ATP in adipose and other tissue preparations incubated with medium containing [32P]phosphate. Biochim Biophys Acta. 1986 Feb 21;885(2):195–205. doi: 10.1016/0167-4889(86)90089-3. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., White M. F. The insulin receptor and the molecular mechanism of insulin action. J Clin Invest. 1988 Oct;82(4):1151–1156. doi: 10.1172/JCI113711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- King M. J., Sale G. J. Dephosphorylation of insulin-receptor autophosphorylation sites by particulate and soluble phosphotyrosyl-protein phosphatases. Biochem J. 1990 Feb 15;266(1):251–259. doi: 10.1042/bj2660251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshio O., Akanuma Y., Kasuga M. Identification of a phosphorylation site of the rat insulin receptor catalyzed by protein kinase C in an intact cell. FEBS Lett. 1989 Aug 28;254(1-2):22–24. doi: 10.1016/0014-5793(89)81001-4. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Grémeaux T., Ballotti R., Van Obberghen E. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resistant obese mice. Nature. 1985 Jun 20;315(6021):676–679. doi: 10.1038/315676a0. [DOI] [PubMed] [Google Scholar]
- Lewis R. E., Cao L., Perregaux D., Czech M. P. Threonine 1336 of the human insulin receptor is a major target for phosphorylation by protein kinase C. Biochemistry. 1990 Feb 20;29(7):1807–1813. doi: 10.1021/bi00459a020. [DOI] [PubMed] [Google Scholar]
- Lewis R. E., Wu G. P., MacDonald R. G., Czech M. P. Insulin-sensitive phosphorylation of serine 1293/1294 on the human insulin receptor by a tightly associated serine kinase. J Biol Chem. 1990 Jan 15;265(2):947–954. [PubMed] [Google Scholar]
- Maegawa H., McClain D. A., Freidenberg G., Olefsky J. M., Napier M., Lipari T., Dull T. J., Lee J., Ullrich A. Properties of a human insulin receptor with a COOH-terminal truncation. II. Truncated receptors have normal kinase activity but are defective in signaling metabolic effects. J Biol Chem. 1988 Jun 25;263(18):8912–8917. [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Lee J., Dull T. J., Ulrich A., Olefsky J. M. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987 Oct 25;262(30):14663–14671. [PubMed] [Google Scholar]
- Morgan D. O., Ho L., Korn L. J., Roth R. A. Insulin action is blocked by a monoclonal antibody that inhibits the insulin receptor kinase. Proc Natl Acad Sci U S A. 1986 Jan;83(2):328–332. doi: 10.1073/pnas.83.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., Roth R. A. Acute insulin action requires insulin receptor kinase activity: introduction of an inhibitory monoclonal antibody into mammalian cells blocks the rapid effects of insulin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):41–45. doi: 10.1073/pnas.84.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S. S., Kahn C. R. Insulin resistance: a look at the role of insulin receptor kinase. Diabet Med. 1988 Oct;5(7):621–629. doi: 10.1111/j.1464-5491.1988.tb01069.x. [DOI] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Shargill N. S., Tatoyan A., el-Refai M. F., Pleta M., Chan T. M. Impaired insulin receptor phosphorylation in skeletal muscle membranes of db/db mice: the use of a novel skeletal muscle plasma membrane preparation to compare insulin binding and stimulation of receptor phosphorylation. Biochem Biophys Res Commun. 1986 May 29;137(1):286–294. doi: 10.1016/0006-291x(86)91208-8. [DOI] [PubMed] [Google Scholar]
- Smith D. M., King M. J., Sale G. J. Two systems in vitro that show insulin-stimulated serine kinase activity towards the insulin receptor. Biochem J. 1988 Mar 1;250(2):509–519. doi: 10.1042/bj2500509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavaré J. M., O'Brien R. M., Siddle K., Denton R. M. Analysis of insulin-receptor phosphorylation sites in intact cells by two-dimensional phosphopeptide mapping. Biochem J. 1988 Aug 1;253(3):783–788. doi: 10.1042/bj2530783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies R. S., Ullrich A., McClain D. A. Augmented mitogenesis and impaired metabolic signaling mediated by a truncated insulin receptor. J Biol Chem. 1989 Aug 5;264(22):12820–12825. [PubMed] [Google Scholar]
- Thomas A. P., Halestrap A. P. Computer stimulation of the effects of alpha-cyano-4-hydroxycinnamate on gluconeogenesis from L-lactate in rat liver cells. Biochem J. 1981 Sep 15;198(3):561–564. doi: 10.1042/bj1980561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornqvist H. E., Avruch J. Relationship of site-specific beta subunit tyrosine autophosphorylation to insulin activation of the insulin receptor (tyrosine) protein kinase activity. J Biol Chem. 1988 Apr 5;263(10):4593–4601. [PubMed] [Google Scholar]
- Tornqvist H. E., Gunsalus J. R., Nemenoff R. A., Frackelton A. R., Pierce M. W., Avruch J. Identification of the insulin receptor tyrosine residues undergoing insulin-stimulated phosphorylation in intact rat hepatoma cells. J Biol Chem. 1988 Jan 5;263(1):350–359. [PubMed] [Google Scholar]
- Van Obberghen E., Kowalski A. Phosphorylation of the hepatic insulin receptor: stimulating effect of insulin on intact cells and in a cell-free system. FEBS Lett. 1982 Jul 5;143(2):179–182. doi: 10.1016/0014-5793(82)80094-x. [DOI] [PubMed] [Google Scholar]
- Vargas A. M., Halestrap A. P., Denton R. M. The effects of glucagon, phenylephrine and insulin on the phosphorylation of cytoplasmic, mitochondrial and membrane-bound proteins of intact liver cells from starved rats. Biochem J. 1982 Oct 15;208(1):221–229. doi: 10.1042/bj2080221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Kahn C. R. Cascade of autophosphorylation in the beta-subunit of the insulin receptor. J Cell Biochem. 1989 Apr;39(4):429–441. doi: 10.1002/jcb.240390409. [DOI] [PubMed] [Google Scholar]
- White M. F., Shoelson S. E., Keutmann H., Kahn C. R. A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem. 1988 Feb 25;263(6):2969–2980. [PubMed] [Google Scholar]
- White M. F., Takayama S., Kahn C. R. Differences in the sites of phosphorylation of the insulin receptor in vivo and in vitro. J Biol Chem. 1985 Aug 5;260(16):9470–9478. [PubMed] [Google Scholar]
- Zick Y., Grunberger G., Podskalny J. M., Moncada V., Taylor S. I., Gorden P., Roth J. Insulin stimulates phosphorylation of serine residues in soluble insulin receptors. Biochem Biophys Res Commun. 1983 Nov 15;116(3):1129–1135. doi: 10.1016/s0006-291x(83)80260-5. [DOI] [PubMed] [Google Scholar]
- Zick Y. The insulin receptor: structure and function. Crit Rev Biochem Mol Biol. 1989;24(3):217–269. doi: 10.3109/10409238909082554. [DOI] [PubMed] [Google Scholar]