Abstract
Adeno-associated virus (AAV)-based muscle gene therapy has achieved tremendous success in numerous animal models of human diseases. Recent clinical trials with this vector have also demonstrated great promise. However, to achieve therapeutic benefit in patients, large inocula of virus will likely be necessary to establish the required level of transgene expression. For these reasons, efforts aimed at increasing the efficacy of AAV-mediated gene delivery to muscle have the potential for improving the safety and therapeutic benefit in clinical trials. In the present study, we compared the efficiency of gene delivery to mouse muscle cells for recombinant AAV type 2 (rAAV-2) and rAAV-2cap5 (AAV-2 genomes pseudo-packaged into AAV-5 capsids). Despite similar levels of transduction by these two vectors in undifferentiated myoblasts, pseudotyped rAAV-2cap5 demonstrated dramatically enhanced transduction in differentiated myocytes in vitro (>500-fold) and in skeletal muscle in vivo (>200-fold) compared to rAAV-2. Serotype-specific differences in transduction efficiency did not directly correlate with viral binding to muscle cells but rather appeared to involve endocytic or intracellular barriers to infection. Furthermore, application of this pseudotyped virus in a mouse model of Duchenne's muscular dystrophy also demonstrated significantly improved transduction efficiency. These findings should have a significant impact on improving rAAV-mediated gene therapy in muscle.
Muscle-based gene therapy protocols have been widely investigated for inherited muscle diseases such as muscular dystrophies. Muscle has also been explored as a platform to produce secreted therapeutic proteins such as factor IX. Among the nonviral and viral vectors used in muscle gene transfer, recombinant adeno-associated virus type 2 (rAAV-2) vectors are especially attractive because they can support persistent transgene expression in muscle. However, clinical studies with rAAV-2 have suggested that technical limitations in viral titer may present a significant hurdle for delivering sufficient levels of virus to completely correct functional defects in patients (17). It is also not entirely clear whether dose escalation of rAAV-2 in clinical trials will achieve the therapeutic goals or whether acute inflammatory responses to viral coat proteins at high doses will uncover risks similar to those encountered in adenoviral trials (29).
To circumvent these problems, various strategies have been under development to enhance the potency of rAAV-2 vectors for in vivo use. Hagstrom and colleagues have demonstrated higher levels of factor IX production from rAAV-2 vectors by modifying the transgene expression cassette (16). We have also been able to achieve a >200-fold enhancement in rAAV-mediated transgene expression in muscle by coadministrating a second super-enhancer vector (12). Supplementing these viral-genome-directed approaches, a wide panel of small chemical compounds has been examined in efforts to identify additional means of improving rAAV-2-mediated gene transfer. For example, dephosphorylation of the single-stranded D-sequence binding protein has been correlated with the activation of rAAV-2 transduction, and in this context a series of tyrosine kinase inhibitors has been developed to increase rAAV-2 transduction by enhancing gene conversion (22). Additionally, in an effort to overcome barriers to intracellular trafficking of rAAV-2, we have also demonstrated a dramatic increase in rAAV-2 transduction in polarized airway cells when proteasome inhibitors are coadministered with the virus (13). Taken together, these different approaches have significantly improved the efficiency of gene transfer with current AAV vectors.
Recently, the cloning and characterization of additional AAV serotypes have provided other potential avenues for improving rAAV transduction. Six primate isolates of AAV serotypes have been reported. Recombinant viral stocks based on these serotypes have also been constructed (1, 4, 5, 19, 23, 36). Among the various AAV serotypes, AAV-2 has been most extensively tested as a gene therapy vector. Detailed sequence comparisons indicate that the AAV-5 capsid proteins are significantly different from those of the other serotypes. The most divergent regions appear to occur at the exterior surface of the mature virion (1, 4). These differences in AAV-2 and AAV-5 biology suggest that recombinant AAV-5 vectors might have a unique niche in gene therapy applications. For example, AAV-5 likely utilizes a different receptor and/or coreceptor for entering cells, and this altered tropism might enhance viral binding and/or endocytosis in certain cell types. Indeed, distinct transduction profiles for AAV-2 and AAV-5 have been demonstrated in several different cell types, including polarized airway epithelia and neuronal cells in vivo (8, 40; Z. Yan, G. Luxton, R. Zak, and J. F. Engelhardt, unpublished data). A recent study in NOD/SCID mice has also suggested that AAV-5 might be a better vector for muscle than AAV-2 (3). To further extend this finding and better understand the mechanisms responsible for increased transduction of rAAV-5 in muscle, we evaluated muscle transduction of a pseudotyped virus in which rAAV-2 genomes were packaged in AAV-5 capsids (rAAV-2cap5). We hypothesized that this hybrid virus should retain the well-established molecular characteristics of the AAV-2 genome, hence allowing direct determination of the influence of the capsid on the efficiency of rAAV gene delivery to muscle. Our in vitro study in myoblasts and in vivo study in muscle demonstrated that gene delivery by pseudotyped rAAV-2cap5 virus was greatly enhanced over rAAV-2 vectors in differentiated myofibers but not in undifferentiated myoblasts. Interestingly, the enhancement in gene transfer with rAAV-2cap5 virus did not completely correlate with increased viral binding, suggesting that a postentry processing event is likely affected by the different capsid structures of AAV-2 and AAV-5. These findings suggest that the intracellular processing of rAAV-2 might also represent a partial barrier to rAAV-2 transduction in muscle, as is seen in other tissues such as the airway.
MATERIALS AND METHODS
Recombinant AAV production.
rAAV-2 virus expressing enhanced green fluorescent protein (EGFP) was generated using the previously described pcisEGFPori3 proviral plasmid (11). The proviral plasmid pcisRSV.Luciferase, which encodes the Rous sarcoma virus (RSV)-driving luciferase gene, was generated by two-step cloning. First, a 1-kb blunted SalI fragment from pREP4 (Invitrogen) was inserted into the blunted XbaI backbone of pSub201 to generate pDD1 (25). Second, a 1.7-kb KpnI/XbaI fragment from pGL3Basic (Promega) was inserted into a KpnI/NheI site in pDD1 to generate pcisRSV.Luciferase. Two helper plasmids (pAV5-Trans and pAV2-Rep) were used to package the AAV-2 genome into the AAV-5 capsid (Z. Yan et al., unpublished data). Briefly, the AAV-5 coding regions (Cap and Rep) were amplified from AAV-5 viral DNA using PCR (1). pAV5-Trans was generated by replacing AAV-2 Cap and Rep genes in pAAV/Ad with a 4.3-kb fragment containing the AAV-5 Cap and Rep genes (24). pAV2-Rep was generated by deleting the AAV-2 Cap gene in pAAV/Ad (24).
rAAV-2 viral stocks were prepared according to a previously described three-plasmid transfection adenovirus-free protocol (37). Briefly, 60% confluent 293 cells were cotransfected with a proviral plasmid (pcisEGFPori3 or pcisRSV.Luciferase), AAV-2 helper plasmid (pXX-2), and adenoviral helper plasmid (pXX6-80) in a ratio of 1:1:3 (9). The crude viral lysate was purified on a Poros heparin column (PerSeptive; Applied Biosystems) using a Beckman Biosys 2000 HPLC workstation and a linear NaCl gradient. The dominant A280 peak fractions (AAV fractions) were pooled and dialyzed against HEPES buffer (20 mM HEPES, 150 mM NaCl, pH 7.8), and stored in aliquots at −80°C in 5% glycerol. Typical yields were approximately 5 × 1012 DNA particles for a twenty 150-mm-diameter plate preparation. Contamination with wild-type AAV-2 was determined as previously described and was less than one functional particle per 1 × 1010 rAAV particles (39).
Pseudotyped rAAV-2cap5 virus (rAAV-2 genomes packaged in AAV-5 capsids) were generated using a modified adenovirus-free system. Briefly, 60% confluent 293 cells were cotransfected with the proviral plasmid (pcisEGFPori3 or pcisRSV.Luciferase), AAV-2 Rep plasmid (pAV2-Rep), AAV-5 helper plasmid (pAV5-Trans), and adenoviral helper plasmid (pXX6-80) in a ratio of 1:1:1:3. Crude viral lysate was purified through three rounds of CsCl equilibrium isopycnic centrifugation as previously described for rAAV-2 (9). Typical yields from this preparation were approximately 5 × 1012 DNA particles for a 150-mm-diameter 20-plate preparation. The physical titer of the viral stock was determined by slot blot hybridization against plasmid standards as previously described (9). Wild type AAV-2/5 hybrid contamination was evaluated by DNA PCR for Rep and Cap genes. Briefly, the viral stock was digested with proteinase K at 37°C for 30 min. Nested PCR was then performed using AAV-5 Cap and Rep gene-specific primer sets. Less than one particle of the wild-type hybrid virus was detected in 1010 pseudotyped viral particles (limits of sensitivity) as determined relative to plasmid Rep and Cap standards.
To confirm that encapsidation of rAAV-2 genome in AAV-5 capsid did not alter the molecular characteristics of the rAAV-2 genome, we performed several control experiments using AAV carrying the cytomegalovirus (CMV)-EGFP expression cassette. First, induction of Rfm (the replication form monomer) and Rfd (the replication form dimer) were equivalent for both rAAV-2 and rAAV-2cap5 virus in the presence of Ad.dl802 coinfection (data not shown). Ad.dl802 coinfection also induced EGFP expression from rAAV-2 and rAAV-2cap5 virus to a similar extent. Second, using a previously described bacterial rescue assay (11), circular monomers and multimers with similar molecular structures were identified in HeLa cells infected with either rAAV-2 or rAAV-2cap5 virus (data not shown).
Recombinant AAV transduction in C2C12 cells.
The C2C12 muscle cell line was obtained from the American Type Culture Collection (catalog number CRL-1772). The cells were cultured in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U of penicillin G/ml, and 100 μg of streptomycin/ml and maintained at 37°C in an incubator with 5% CO2. Differentiation was induced by culturing the cells in 10% horse serum (Z. Yan et al., unpublished data). Infections were performed in serum-free DMEM for the indicated amount of time specified in each experiment. When required, 20% FBS–DMEM was added 2 h after infection to bring the final serum level to 10%. In the case of heparin competition experiments, viruses were preincubated with free heparin (20 μg/ml; Sigma) for 60 min on ice, and infections were then carried out in serum-free medium containing free heparin (final concentration, 20 μg/ml) (31). To study the effect of sialic acid on rAAV binding, C2C12 cells were first rinsed with serum-free DMEM and then incubated with type III neuraminidase (sialidase) (catalog number N7885; Sigma-Aldrich) at a final enzyme concentration of 200 mU/ml in serum-free medium for 2 h at 37°C. The C2C12 cells were then washed with serum-free DMEM before viral inoculation (20, 32). To analyze the effect of the proteasome inhibitor on rAAV transduction, the indicated amount of viral particles was applied to the C2C12 cells in the presence or absence of proteasome inhibitors in serum-free medium. Tripeptide proteasome inhibitors N-acetyl-l-leucyl-l-leucyl-norleucine (LLnL) and benzyloxycarbonyl-Leu-Leu-l-leucinal (Z-LLL) were purchased from Calbiochem-Novabiochem Corporation (La Jolla, Calif.). At 1 h postinfection, the final serum concentration was increased to 10% by the additional FBS. Both virus and proteasome inhibitors were removed from cells at 4 h postinfection. Transgene expression was quantified at 24 h postinfection.
Analysis of rAAV transduction in C2C12 cells.
The efficiency of rAAV transduction in C2C12 cells was monitored by the level of EGFP or luciferase transgene expression. EGFP expression was monitored by fluorescence microscopy, and luciferase expression was determined using a previously published protocol at a measuring sensitivity of 75% (12). To evaluate viral binding and persistence in C2C12 cells, the low-molecular-weight Hirt DNA was harvested at the indicated times following viral infection. DNA samples were then resolved in a 0.8% agarose gel and blotted onto a Hybond N+ nylon membrane as described previously (10). Each lane represents the DNA from one 35-mm-diameter-plate cell culture. The viral genomes were detected with a transgene-specific probe at 106 cpm/ml and washed at a stringency of 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate at 60°C for 20 min.
Detection of α-2,3-linked sialic acid expression in C2C12 cells.
C2C12 cells were plated on sterile positively charged glass slides at a concentration of 2 × 105 cells/slide, and differentiation was induced as described above. Maackia amurensis lectin II (MAL II) binding assays were performed by first chilling the cells at 4°C for 10 min in serum-free medium. The cultures were then incubated with biotinylated MAL II (catalog number B-1265; Vector Laboratories Inc. ) at 4°C for 30 min. After three washes with serum-free DMEM, cells were fixed with 4% paraformaldehyde in phosphate-buffed saline (PBS). Following fixation, the cells were rinsed with HEPES buffer and then incubated with fluorescein isothiocyanate (FITC)-conjugated avidin at room temperature for 15 min. Finally, cells were mounted with Citifluo antifadent and the amount of cell surface α-2,3-linked sialic acid was determined by indirect fluorescent microscopy.
Evaluating rAAV transduction in murine skeletal muscle.
Snj/ScSn mice were purchased from Jackson Laboratory. Snj mice are a normal BL10 strain. ScSn mice (mdx) have a spontaneous mutation in exon 23 of the dystrophin gene and do not express murine dystrophin (2). Since the dystrophic phenotype is manifested only in adult mice, we used 6-month-old mice in our study. The delivery of rAAV to the anterior tibialis was performed according to a previously published protocol (11). To decrease intermouse variability, the left anterior tibialis muscle of each mouse was infected with 2 × 1010 particles of rAAV-2cap5 virus, and the right anterior tibialis muscle of the same mouse was infected with 2 × 1010 particles of rAAV-2. EGFP expression was determined either in freshly isolated muscles or in 15-μm-thick cryosections from paraformaldehyde-fixed tissues. To visualize the pathological changes in mdx mouse muscle, mice were infused with 400 μl of Evans blue dye (10 mg/ml) through the tail vein at 5 h prior to tissue harvest. To facilitate contraction-induced muscle injury and dye diffusion, mice were exercised by swimming twice for 10 min at 30-min intervals during the first hour following dye injection. Muscle luciferase levels, following infection with 2 × 1010 particles per muscle of luciferase-expressing rAAV-2 or rAAV-2cap5, were analyzed as described previously (12).
RESULTS
Encapsidation of rAAV-2 genome in AAV-5 capsid enhances transduction in differentiated, but not undifferentiated, C2C12 cells.
C2C12 cells are myoblast cells derived from the C3H strain of mice which can differentiate into contractile myotubes and produce muscle-specific proteins. In undifferentiated C2C12 cells, no significant difference in transgene expression was observed with CMV-driving EGFP vectors when the same numbers of DNA particles of rAAV2 or rAAV2cap5 were used for infection (Fig. 1). However, when differentiated C2C12 cells were infected under identical conditions, we observed a dramatic increase in EGFP expression in rAAV2cap5-infected cells but not in rAAV-2-infected cells (Fig. 1). Despite the apparent increase in transgene-expressing cells, quantifying the percentage of EGFP-positive cells yielded little quantitative information on the average increase in transgene expression on a per cell basis. To further characterize the time course of rAAV transduction and exclude promoter- and/or transgene-related artifacts, we repeated our study with vectors containing the RSV promoter-driving luciferase. The use of the luciferase reporter gene also permitted a more sensitive and quantitative analysis. As shown in Fig. 2, low-level transduction was observed in undifferentiated myoblasts for both rAAV-2 and rAAV-2cap5 viruses. Consistent with our findings using CMV-EGFP vectors, rAAV-2-mediated luciferase expression dropped an order of magnitude in differentiated C2C12 cells. In contrast, transgene expression from the rAAV-2cap5 virus was significantly enhanced in well-differentiated myotubes, with a >500-fold increase in luciferase activity in comparison to undifferentiated cells at 72 h postinfection (Fig. 2B). These findings suggest that pseudotyped rAAV-2cap5 virus might prove to be a more efficacious vector for gene delivery to postmitotic myofibers in vivo.
FIG. 1.
Myoblast differentiation increases transduction with rAAV-2cap5 but not rAAV-2 virus. Transduction of undifferentiated (A to D) and differentiated (E to H) C2C12 cells was evaluated for EGFP transgene expression following infection with 3,000 DNA particles/cell of either rAAV-2 (A, B, E, and F) or rAAV-2cap5 (C, D, G and H) virus for 24 h. EGFP expression was evaluated 72 h after infection by fluorescence microscopy. Nomarski and fluorescence photomicrographs are presented to the left and right of each panel, respectively. (I) Quantitative analysis of the percentage of EGFP-expressing cells. Values represent the means ± standard errors of the means (error bars) for more than 15 quantitated 10× magnification microscropic fields from three independent experiments.
FIG. 2.
Quantitative analysis of RSV-luciferase expression from rAAV-2 and rAAV-2cap5 virus in differentiated and undifferentiated C2C12 cells. (A) Undifferentiated and differentiated C2C12 cells were infected with either rAAV-2 or rAAV-2cap5 virus for 24 h at an MOI of 3,000 DNA particles/cell. Mock-infected cells were used as a negative control for background enzyme activity. The luciferase activity was determined at 24, 48, and 72 h after infection. (B) Ratio of relative luciferase expression (rAAV-2cap5/rAAV-2) for the two vector types. Values in both panels represent the means ± standard errors of the means (error bars) for three independent data points.
Differences in viral binding cannot explain the discordance in C2C12 cell transduction with rAAV-2 and rAAV-2cap5 virus.
We next examined whether the different transduction profiles seen in differentiated C2C12 cells were due to differences in viral binding, as might be anticipated by altered capsid structure. Previous studies have also suggested that factors affecting viral endocytosis also influence transgene expression from rAAV vectors (10, 31). To compare the viral binding efficiencies, C2C12 cells (undifferentiated or differentiated) were incubated with rAAV-2 or rAAV-2cap5 virus at 4°C for 60 min. Low-molecular-weight Hirt DNA was harvested from infected cells after washing with PBS or trypsinization to remove extracellular bound virus. The overall viral binding to the cell surface was determined by Southern blotting of Hirt DNA (Fig. 3). Surprisingly, AAV-2 capsid, which provided poor transduction, mediated higher binding efficiency in both undifferentiated and differentiated C2C12 cells than the AAV-5 capsid (Fig. 3, lanes 6 and 12). Furthermore, surface-bound rAAV-2 was easily removed by trypsin (Fig. 3, lanes 5 and 11). In striking contrast, irrespective of the cellular differentiation state, lower levels of the rAAV-2cap5 pseudotyped virus bound to the cell surface compared to rAAV-2 under identical infection conditions. These data suggest that differences in endocytic mechanisms and/or intracellular processing, but not viral binding, must be responsible for the higher level of transduction seen with the pseudotyped virus.
FIG. 3.
Examination of viral binding in C2C12 cells. (A) Viral binding was assessed by Southern blot analysis of viral DNA. C2C12 cells were precooled at 4°C for 10 min. After washing with serum-free DMEM, rAAV-2 (lanes 5, 6, 11, and 12) or rAAV-2cap5 (lanes 2, 3, 8, and 9) viruses (carrying the AAV-2 CMV-EGFP cassette) were applied to the cells at an MOI of 2,000 particles/cell for 60 min at 4°C. Mock-infected cells were included as negative controls (lanes 1, 4, 7, and 10). At the end of incubation, cells were either washed with PBS alone (lanes 1, 3, 4, 6, 7, 9, 10, and 12) or treated with 0.5% trypsin (lanes 2, 5, 8, and 11) before washing. Hirt DNA was then prepared and analyzed by Southern blotting with a transgene (EGFP)-specific 32P-labeled probe. Abbreviations: Mock, mock-infected cells; Pseudo, rAAV-2cap5 virus; AAV-2, native rAAV-2 virus. (B) Viral binding from three independent experiments was quantified by densitometry. Values shown are means ± standard errors of the means (error bars). Lane numbers in panel B correspond to the labeling in panel A.
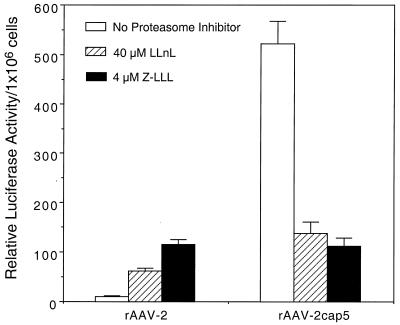
To further dissect potential differences between rAAV-2 and rAAV-2cap5 in intracellular processing, we compared their transduction profiles following treatment with proteasome inhibitors. Tripeptide proteasome inhibitors have recently been shown to enhance persistent rAAV-2 transduction in polarized airway cells. This induction involves alterations in several aspects of viral endocytosis, such as viral ubiquitination, endosomal processing, and nuclear trafficking (13). Therefore, response to proteasome inhibitors may indirectly reflect the molecular mechanisms by which AAV is processed through the endosomal compartment. Fully differentiated C2C12 cells were infected with either rAAV-2 or rAAV-2cap5 at a multiplicity of infection (MOI) of 600 particles/cell (Fig. 4). In the presence of either 40 μM LLnL or 4 μM Z-LLL, rAAV-2 transduction was increased 6- or 10-fold, respectively. Interestingly, application of LLnL or Z-LLL resulted in a significant decrease in transgene expression in rAAV-2cap5-infected cells. These data strongly suggest that rAAV-2 and rAAV-2cap5 follow distinct intracellular pathways following endocytosis in differentiated C2C12 cells.
FIG. 4.
Proteasome inhibitors differentially affect rAAV-2 and rAAV-2cap5 transduction in differentiated C2C12 cells. To analyze the effect of proteasome inhibitors on the intracellular processing of different AAV serotypes, fully differentiated C2C12 cells were infected with either rAAV-2 or rAAV-2cap5 luciferase vectors at an MOI of 600 DNA particles/cell for 4 h. Tripeptide proteasome inhibitors (40 μM LLnL or 4 μM Z-LLL) were also added to the media during the infection period. Luciferase expression was quantified at 24 h postinfection. The data represent the means ± standard errors of the means (error bars) for three independent samples for each experimental condition.
Southern blot analysis also revealed another interesting aspect of AAV-5 capsid binding. In our protocol, trypsinization was initially used to confirm that the viral particles were not internalized during the 4°C incubation (10, 13). Two assumptions were made in this study. First, it was assumed that the plasma membrane is inert and lacks active endocytosis at 4°C. Second, it was assumed that stringent trypsinization (0.5% trypsin) should remove all surface-bound viral particles. This was indeed the case for rAAV-2 virus in many different cell types, such as HeLa cells (10), primary cultured human airway epithelial cells (13), and C2C12 cells (Fig. 3). Unexpectedly, a significant amount of trypsin-resistant viral DNA was detected in rAAV-2cap5 virus-infected C2C12 cells. These data indicate either that a very efficient and/or fast internalization of AAV-5 capsid occurred or that the interaction between the AAV-5 capsid and its receptor has a very high affinity and/or is relatively trypsin insensitive.
Increased transduction of rAAV-2cap5 pseudotyped virus in differentiated C2C12 cells correlates with increased viral binding.
Information gained from viral binding studies at 4°C also shed light on why differentiation of C2C12 cells leads to significant increases in transduction with rAAV-2cap5 virus. Consistent with increased transgene expression, an eightfold increase in viral binding was observed for rAAV-2cap5 virus in differentiated cells compared to undifferentiated cells (compare lanes 9 and 3 in Fig. 3). However, the magnitude of increased binding was approximately 2 orders of magnitude lower than the increase in transgene expression in differentiated cells (Fig. 2). These findings also suggest that enhanced viral binding of AAV-5 capsids cannot completely explain the increased transduction efficiency seen in differentiated myotubes.
Recently, α-2,3-linked sialic acid was identified as a cellular receptor for rAAV-5 (32). MAL II preferentially binds to α-2, 3-linked sialic acid and hence can be used to assess the abundance of this sialic acid form. To further characterize the enhanced binding of rAAV-2cap5 pseudotyped virus in differentiated C2C12 cells, we examined the MAL II binding pattern in both undifferentiated and differentiated cells. Consistent with the viral binding profile, cell surface expression of α-2,3- linked sialic acid was significantly upregulated in differentiated cells as indicated by enhanced MAL II binding (Fig. 5).
FIG. 5.
The AAV-5 receptor is upregulated following differentiation of C2C12 cells. To correlate increased transduction of rAAV-2cap5 in differentiated C2C12 cells with AAV-5 receptors, cell surface α-2,3-linked sialic acid expression was determined using a MAL II lectin binding assay. MAL II lectin binding was visualized in undifferentiated (A and B) and differentiated (C and D) C2C12 cells using indirect avidin-FITC fluorescence microscopy (B and D). (A and C) Nomarski photomicrographs of panels B and D, respectively. Increased AAV-5 receptor expression in fully differentiated cells is clearly demonstrated in D.
To further analyze the interaction between sialic acid and the AAV-5 capsid protein, C2C12 cells were pretreated with type III neuraminidase (sialidase). As shown in Fig. 6, sialidase treatment completely abolished the AAV-5 capsid binding to C2C12 cells (Fig. 6, lanes 1 and 7). However, identical treatment had only minimal effects on AAV-2 capsid binding in these cells (Fig. 6, lanes 4 and 10). As a control, we also evaluated the effect of free heparin on viral binding. Heparan sulfate proteoglycan has been reported as the primary attachment receptor for AAV-2 virus (26). Heparan sulfate proteoglycan is also associated with the initial binding of many other viruses, including herpes simplex virus and human immunodeficiency virus (D. Duan, Y. Yue, and J. F. Engelhardt, Letter, Hum. Gene Ther. 10:1553–1557, 1999). Consistent with other reports, preincubation with free heparin dramatically decreased AAV-2 but not AAV-5 capsid binding in C2C12 cells.
FIG. 6.
Factors affecting rAAV binding in C2C12 cells. (A) The effects of heparin competition or sialidase (NA III) treatment on rAAV-2 and rAAV-2cap5 virus infection in C2C12 cells were evaluated. rAAV-2 or rAAV-2cap5 infections (MOI of 1,000 DNA particles/cell) of undifferentiated (lanes 1 to 6) or differentiated (lanes 7 to 12) C2C12 cells were evaluated following no treatment (lanes 3, 6, 9, and 12), sialidase treatment (lanes 1, 4, 7, and 10), or heparin (final concentration, 20 μg/ml) competition (lanes 2, 5, 8, and 11). Hirt DNA was harvested after incubation at 4°C for 60 min and evaluated by Southern blotting against a 32P-labeled EGFP probe. Pseudo, rAAV-2cap5 virus. (B) Results from densitometric quantification of DNA signals from three independent experiments. NAIII, type III neuraminidase (sialidase). Values are represented as percent inhibition (means ± standard errors of the means [error bars]; n = 3) in binding following sialidase treatment or heparin competition compared to untreated controls.
Serotype-specific capsid entry pathways affect the stability of viral genomes following infection.
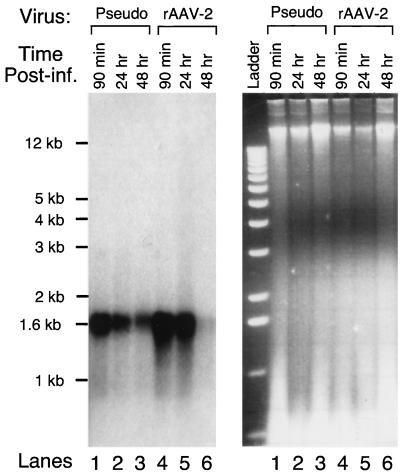
As discussed above, the difference in the intracellular processing appears to be a determining factor for the diverse transduction profiles between rAAV-2 and rAAV-2cap5 pseudotyped virus in fully differentiated C2C12 cells. To further characterize this process, we analyzed the kinetics of viral genome persistence with these two recombinant vectors (Fig. 7). Important to this analysis is the fact that the two recombinant viruses differ by only their capsid structures and contain identical viral genomes. Differentiated C2C12 cells were infected at the same particle MOI with either rAAV-2 or rAAV-2cap5 at 4°C for 90 min. Hirt DNA was prepared either immediately following infection at 4°C or at 24 and 48 h following a shift to 37°C. Consistent with the findings in Fig. 3 and 6, rAAV-2 virus attached to differentiated C2C12 cells more efficiently during the 90-min incubation at 4°C. However, by 48 h postinfection at 37°C, the intracellular level of single-stranded viral genomes delivered by AAV-2 capsid dropped to almost undetectable levels. Interestingly, the viral genomes introduced by the AAV-5 capsid were significantly more stable. Since the only difference between pseudotype virus and the rAAV-2 was the viral capsid, we hypothesize that different pathways for processing internalized AAV-2 and AAV-5 viral-capsid-encoded genomes affect viral genome persistence. However, it should also be stressed that the 1.6-kb single-stranded viral genome is not directly responsible for transgene expression. Nonetheless, these genomes are precursors for genome conversion to a transgene-expressible form, and hence the stability of single-stranded DNA viral genomes will likely affect the extent to which virus can ultimately express an encoded transgene. It remains to be determined whether the double-stranded transcriptionally active proviral genomes are also differentially regulated.
FIG. 7.
Kinetic analysis of rAAV viral genome persistence in differentiated C2C12 cells. To better understand rAAV transduction in myotubes, differentiated C2C12 cells were infected with either rAAV-2cap5 (lanes 1, 2, and 3) or rAAV-2 (lanes 4, 5, and 6) at an MOI of 1,000 DNA particles/cell. Hirt DNA was harvested at 90 min (lanes 1 and 4), 24 h (lanes 2 and 4), and 48 h (lanes 3 and 6) postinfection. The left panel depicts a Southern blot hybridized with a 32P-labeled EGFP probe. The right panel depicts the corresponding ethidium bromide-stained gel. The lane labels in both panels are identical with the exception of the DNA ladder. Pseudo, rAAV-2cap5 virus.
AAV-5 capsids mediate increased transduction of normal and dystrophic muscle.
To further expand our in vitro findings, we examined the transduction efficiency of both pseudotyped rAAV-2cap5 and native rAAV-2 in mouse skeletal muscle. Two sets of experiments were carried out with viruses harboring either a CMV-EGFP or an RSV-luciferase expression cassette. Transgene expression was evaluated at 1 week and 1 month after infection.
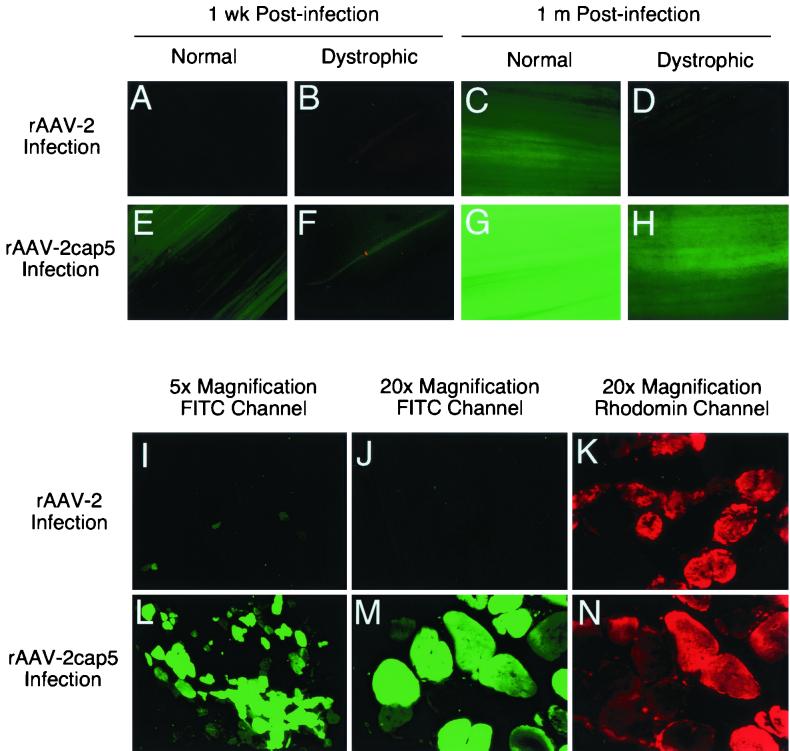
Consistent with our previous report (11), rAAV-2-mediated EGFP expression was barely detectable at 1 week postinfection in normal muscle (Fig. 8A). In sharp contrast, at 1 week postinfection, a significantly higher level of EGFP expression was detected in normal muscle infected with rAAV-2cap5 virus (Fig. 8E). Evaluation of the transgene expression 1 month after infection also demonstrated a much higher EGFP expression in normal muscle infected with rAAV-2cap5 compared to rAAV-2 (Fig. 8G and C).
FIG. 8.
Kinetic comparison of EGFP expression in normal and dystrophic muscles. The anterior tibialis muscles of 6-month-old normal or mdx mice were infected with 2 × 1010 particles of the indicated viruses as described in Materials and Methods. EGFP expression was determined at different time points by fluorescence microscopy. (A to H) Photographs of whole-mount tissue from freshly excised muscles 1 wk and 1 month after infection. Representative photographs from triplicate experiments are shown. Photomicrographs were taken at an 8-s (A, B, E, and F) or 1-s (C, D, G, and H) exposure. (I to N) EGFP expression 6 months after infection of mdx tibialis muscles was evaluated in paraformaldehyde-fixed, cryopreserved tissue sections (15 μm thick) following Evans blue perfusion to demarcate damaged myofibers. Photomicrographs in panels I to K (rAAV-2 infection) were taken from the right leg, and those in panels L and N (rAAV-2cap5 infection) were taken from the left leg of the same mouse. Photomicrographs were taken at 15-s (I and L) or 2-s (J, K, M, and N) exposures. FITC photomicrographs are represented (I, J, L, and M). Panels J and M (FITC channel) are identical to fields shown in panels K and N (Evans blue, rhodamine channel), respectively.
A previous report has suggested that rAAV-2 transduction in dystrophic muscle may be significantly decreased due to the disease process (6). We sought to determine whether pseudotyped rAAV-2cap5 virus might impart some level of increased transduction in diseased mdx skeletal muscle. As seen in normal muscle, rAAV-2cap5 infection afforded significantly higher levels of transduction in mdx muscles (Fig. 8F and H) than did native rAAV-2 virus infection (Fig. 8B and D). However, the level of rAAV-mediated EGFP expression was significantly reduced in mdx mice infected with either rAAV-2cap5 or rAAV-2 virus compared to that in normal control littermates (Fig. 8A to H). EGFP expression in dystrophic muscle was also examined at 6 months postinfection. Consistent with the 1-week and 1-month findings, prominent EGFP expression was found only in rAAV-2cap5-infected muscle samples (Fig. 8I toN). Very few EGFP-positive myofibers were detected in rAAV-2-infected muscles. Furthermore, the intensity of EGFP expression in each individual myofiber was also much lower in the rAAV-2 infection group. Of interest, Evans blue-positive, damaged myofibers appeared to be transduced by rAAV-2cap5 at an equal efficiency to nondamaged Evans blue-negative myofibers (Fig. 8J, K, M, and N).
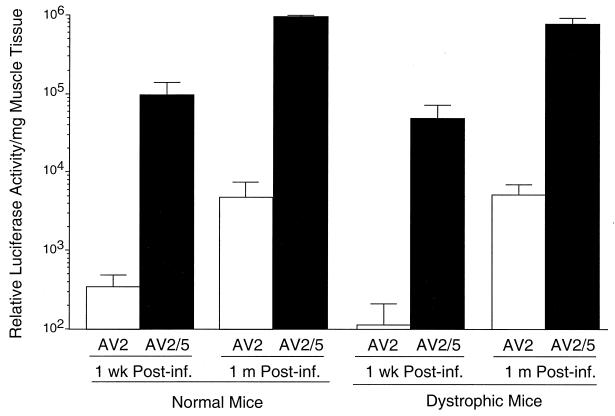
In an effort to obtain a more quantitative understanding of the transduction profiles in normal and dystrophic muscles, viruses carrying the more-sensitive RSV-luciferase expression cassette were used. As demonstrated in Fig. 9, rAAV-2cap5 virus infection resulted in a >200-fold enhancement in luciferase expression at 1 week and 1 month postinfection compared to native rAAV-2 virus. Surprisingly, a similar profile of enhancement was achieved in both normal and dystrophic muscle. Several aspects of the reporter gene and/or the methods used for detection could have potentially influenced the discordance in dystrophic muscle expression of EGPF and/or luciferase reporters. These include the half-life, the immunogenicity of the transgene products in the setting of diseased myofibers, and the sensitivity of the transgene expression assays (minimal threshold and maximal saturating levels for detection). Luciferase is very sensitive to protease degradation, and in transfected mammalian cells, its half-life is about 3 h (27). In contrast, GFP is extremely stable and has a longer half-life (34). Therefore, it is unlikely that disease-induced alterations in the degradation of the reporter proteins can explain our observations. Previous study has suggested that immunoreactivity of a transgene-encoded protein is a critical determinant for the stability of transgene expression in immunocompetent mice (28). Hence, it is plausible that in the setting of Duchenne's muscular dystrophy, EGFP may be more immunogenic than luciferase. Despite these potential issues with the immunogenicity of EGFP and luciferase, our data clearly demonstrated that rAAV-2cap5 pseudotyped virus was much more effective (>200-fold) in transducing both normal and mdx skeletal muscle. Given the identity of the viral genomes in both native rAAV-2 and pseudotyped rAAV-2cap5 virus, these findings implicate AAV-5 capsid interactions with myofibers as the sole determinant for increased transduction.
FIG. 9.
Quantitative examination of luciferase activity following rAAV-2cap5 or rAAV-2 infection of tibialis muscles. rAAV luciferase expression vectors were used to evaluate transgene expression in normal and mdx anterior tibialis muscles at 1 week and 1 month postinfection with 2 × 1010 particles of rAAV-2 (AV2) or rAAV-2cap5 (AV2/5). The data represent the means ± standard errors of the means (error bars) for three independent muscle samples from each experimental group.
DISCUSSION
In this study, we have examined the transduction efficiency of identical rAAV-2 genomes delivered by two different viral capsids. This capsid modification strategy has been extensively used by many researchers to either direct targeted expression or improve the transduction efficiency for certain cells which are less transducible with rAAV-2 (14, 35). The rationale for our study was based on the recent findings that rAAV-5 can significantly enhance rAAV-mediated gene transfer in certain cell types (8, 40). Since the homology for both viral inverted terminal repeats and capsid proteins is only about 60% between AAV-2 and AAV-5, it is conceivable that either the viral genome or the capsid structure could be responsible for the improved transduction efficiency with rAAV-5. To better understand the functional contribution of the viral capsid alone, we utilized a hybrid viral system in which rAAV-2 genomes were packaged in AAV-5 capsids. This pseudotyped virus should comparatively eliminate any contributions of the viral genome to transduction efficiency. Both in vitro studies in differentiated cells and in vivo data in mouse skeletal muscle indicated that pseudotyped virus was significantly more efficient in mediating transgene expression than native rAAV-2 virus.
One unexpected finding was that the transduction efficiency of rAAV was significantly affected by the cellular state of differentiation in C2C12 cells. Furthermore, the influence of differentiation had opposite effects for the two serotypes of rAAV analyzed. In the case of rAAV-2 infection, differentiation of C2C12 cells decreased viral transduction by 10-fold. In contrast, differentiation increased transgene expression with pseudotyped rAAV-2cap5 virus by more than 500-fold. The differentiation of the myoblasts into contractile myotubes involves the coordinated expression of many cellular factors. When growth factors are deprived (as is the case when inducing differentiation of C2C12 cells), the proliferating myocytes enter a terminal differentiation stage and start to express various differentiation factors (such as myogenin and p21/WAF1) and contractile proteins (such as myosin and troponin) (30). It is presently not clear what factors are directly linked to the enhanced transduction of differentiated cells by pseudotyped virus. However, our data do suggest that the differentiation-associated changes in cell surface lectin expression contribute to the increased viral binding of AAV-5 capsids to myotubes following pseudotyped virus infection. Nevertheless, binding of rAAV to the cell surface appeared not to be the primary determinant of differences in the transduction efficiency between rAAV-2 and rAAV-2cap5 viruses. The overall attachment of rAAV-2cap5 to muscle cells was weaker than that for rAAV-2. Furthermore, unlike rAAV-2, transduction of differentiated myotubes with pseudotyped rAAV-2cap5 virus was negatively regulated by proteasome inhibitors. We currently hypothesize that differentiation-induced changes in the intracellular characteristics of myotubes might be a more important factor contributing to the higher level of transduction with rAAV-2cap5. In this context, several possibilities might account for our findings. For example, muscle differentiation might enhance intracellular processing and/or uncoating of incoming pseudotyped virions. Alternatively, cellular differentiation could also adversely effect the intracellular movement of AAV-2 capsid packaged virions and lead to lower transduction. Furthermore, differentiation might alter the rate of internalization of AAV-5 but not AAV-2 receptors at the membrane. Future studies evaluating intracellular processing of AAV will be needed to distinguish between these potential mechanisms.
The results from in vivo analyses comparing rAAV-2 to rAAV-2cap5 virus were also quite interesting. Several previous reports have suggested that rAAV-2 efficiently transduces dystrophic skeletal muscle and produces high levels of therapeutic proteins, including different sarcoglycans and microdystrophin (7, 15, 18, 33). However, recent studies also suggest that rAAV-2-mediated transgene expression is significantly reduced in dystrophic muscle if the transgene is driven by a ubiquitous viral promoter (6). This has been attributed to ectopic transgene expression in antigen-presenting cells and subsequent immune clearance of the transgene-expressing cells. Our studies evaluating rAAV-mediated RSV-luciferase gene delivery demonstrated little difference in gene expression between normal and mdx muscles. However, results evaluating rAAV-mediated EGFP expression in mdx mice were quite different. In our study, despite a decreased EGFP expression in rAAV-2 infected mdx muscle, high-level transduction was observed following infection with rAAV-2cap5 pseudotyped virus (Fig. 8I to N). Although rAAV-2cap5-mediated EGFP gene expression was lower in mdx than in normal muscles, compared with rAAV-2, there appeared to be a lower degree of disease-associated effects on transgene expression with rAAV-2cap5 virus. Since different capsid structures determine the dissimilar cellular tropisms of AAV-2 and AAV-5 (8, 40), differences in disease-associated effects on rAAV-2 and rAAV-2cap5 EGFP expression might be explained by a decreased susceptibility of dendritic cells to AAV-5 infection. Further investigation in this area is warranted.
Recent studies have suggested that rAAV-2 is capable of circumventing the maturation-dependent barrier of muscle gene transfer by other viruses, including adenovirus, retrovirus, and herpesvirus (21). Since myofiber maturation and myoblast differentiation represent distinct biological processes, it remains to be determined whether AAV-5 capsid can provide additional benefits in overcoming this barrier. It has also been suggested that rAAV-2 preferentially transduces type I slow myofiber, and this propensity might be associated with the overexpression of the rAAV-2 receptor heparan sulfate proteoglycan. Further examination of potential myofiber subtype preferences for AAV-5 capsid infection may uncover further mechanistic insights into how AAV-5 pseudotyping increases transduction in differentiated muscle.
In summary, these studies have begun to shed light on biological differences between AAV-2 and AAV-5 capsids and their effect on cell-vector interactions in muscle cells. Differences in the biology of viral infectious processes between these two vectors significantly affect their efficiency of delivering transgenes into differentiated myofibers. Interestingly, skeletal muscle has been traditionally thought to lack many of the barriers to rAAV-2 infection seen in other tissues, such as the airway. However, studies comparing rAAV-2 and rAAV-2cap5 suggest that muscle may also have similar barriers to rAAV-2 infection involving endocytosis and/or intracellular processing that limit its full utility as a gene therapy vector. In this context, a principle lesson from these studies is that the efficiency of viral binding does not always directly correlate with transduction efficiency. This is not entirely surprising given the reported influences of the coreceptor(s) in endocytosis of rAAV vectors. Studies evaluating phenotypic differences induced by myoblast differentiation may begin to shed more light on the cellular factors controlling the efficiency of AAV endocytosis and/or intracellular processing.
ACKNOWLEDGMENTS
We thank Robert Walters and Joseph Zabner for critical review of the manuscript. We gratefully acknowledge Terry Ritchie for editorial assistance in preparation of the manuscript.
This work was supported by NIH grant HL58340 (J.F.E. and D.D.) and the Center for Gene Therapy is funded by NIH (P30 DK54759) (J.F.E.) and the Cystic Fibrosis Foundation. J.F.E. and D.D. are also supported by a research grant from the Muscular Dystrophy Association.
REFERENCES
- 1.Bantel-Schaal U, Delius H, Schmidt R, zur Hausen H. Human adeno-associated virus type 5 is only distantly related to other known primate helper-dependent parvoviruses. J Virol. 1999;73:939–947. doi: 10.1128/jvi.73.2.939-947.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulfield G, Siller W G, Wight P A, Moore K J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao H, Liu Y, Rabinowitz J, Li C, Samulski R J, Walsh C E. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- 4.Chiorini J A, Kim F, Yang L, Kotin R M. Cloning and characterization of adeno-associated virus type 5. J Virol. 1999;73:1309–1319. doi: 10.1128/jvi.73.2.1309-1319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiorini J A, Yang L, Liu Y, Safer B, Kotin R M. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J Virol. 1997;71:6823–6833. doi: 10.1128/jvi.71.9.6823-6833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordier L, Gao G P, Hack A A, McNally E M, Wilson J M, Chirmule N, Sweeney H L. Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies. Hum Gene Ther. 2001;12:205–215. doi: 10.1089/104303401750061267. [DOI] [PubMed] [Google Scholar]
- 7.Cordier L, Hack A A, Scott M O, Barton-Davis E R, Gao G-P, Wilson J M, McNally E M, Sweeney H L. Rescue of skeletal muscles of r-sarcoglycan deficient mice with adeno-associated virus-mediated gene transfer. Mol Ther. 2000;1:119–129. doi: 10.1006/mthe.1999.0019. [DOI] [PubMed] [Google Scholar]
- 8.Davidson B L, Stein C S, Heth J A, Martins I, Kotin R M, Derksen T A, Zabner J, Ghodsi A, Chiorini J A. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci USA. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan D, Fisher K J, Burda J F, Engelhardt J F. Structural and functional heterogeneity of integrated recombinant AAV genomes. Virus Res. 1997;48:41–56. doi: 10.1016/s0168-1702(96)01425-6. [DOI] [PubMed] [Google Scholar]
- 10.Duan D, Li Q, Kao A W, Yue Y, Pessin J E, Engelhardt J F. Dynamin is required for recombinant adeno-associated virus type 2 infection. J Virol. 1999;73:10371–10376. doi: 10.1128/jvi.73.12.10371-10376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan D, Sharma P, Yang J, Yue Y, Dudus L, Zhang Y, Fisher K J, Engelhardt J F. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long term episomal persistence in muscle. J Virol. 1998;72:8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan D, Yue Y, Yan Z, Engelhardt J F. A new dual-vector approach to enhance recombinant adeno-associated virus-mediated gene expression through intermolecular cis activation. Nat Med. 2000;6:595–598. doi: 10.1038/75080. [DOI] [PubMed] [Google Scholar]
- 13.Duan D, Yue Y, Yan Z, Yang J, Engelhardt J F. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Investig. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girod A, Ried M, Wobus C, Lahm H, Leike K, Kleinschmidt J, Deleage G, Hallek M. Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2. Nat Med. 1999;5:1052–1056. doi: 10.1038/12491. [DOI] [PubMed] [Google Scholar]
- 15.Greelish J P, Su L T, Lankford E B, Burkman J M, Chen H, Konig S K, Mercier I M, Desjardins P R, Mitchell M A, Zheng X G, Leferovich J, Gao G P, Balice-Gordon R J, Wilson J M, Stedman H H. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nat Med. 1999;5:439–443. doi: 10.1038/7439. [DOI] [PubMed] [Google Scholar]
- 16.Hagstrom J N, Couto L B, Scallan C, Burton M, McCleland M L, Fields P A, Arruda V R, Herzog R W, High K A. Improved muscle-derived expression of human coagulation factor IX from a skeletal actin/CMV hybrid enhancer/promoter. Blood. 2000;95:2536–2542. [PubMed] [Google Scholar]
- 17.Kay M A, Manno C S, Ragni M V, Larson P J, Couto L B, McClelland A, Glader B, Chew A J, Tai S J, Herzog R W, Arruda V, Johnson F, Scallan C, Skarsgard E, Flake A W, High K A. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Dressman D, Tsao Y P, Sakamoto A, Hoffman E P, Xiao X. rAAV vector-mediated sarcoglycan gene transfer in a hamster model for limb girdle muscular dystrophy. Gene Ther. 1999;6:74–82. doi: 10.1038/sj.gt.3300830. [DOI] [PubMed] [Google Scholar]
- 19.Muramatsu S, Mizukami H, Young N S, Brown K E. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virology. 1996;221:208–217. doi: 10.1006/viro.1996.0367. [DOI] [PubMed] [Google Scholar]
- 20.Pickles R J, Fahrner J A, Petrella J M, Boucher R C, Bergelson J M. Retargeting the coxsackievirus and adenovirus receptor to the apical surface of polarized epithelial cells reveals the glycocalyx as a barrier to adenovirus-mediated gene transfer. J Virol. 2000;74:6050–6057. doi: 10.1128/jvi.74.13.6050-6057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pruchnic R, Cao B, Peterson Z Q, Xiao X, Li J, Samulski R J, Epperly M, Huard J. The use of adeno-associated virus to circumvent the maturation-dependent viral transduction of muscle fibers. Hum Gene Ther. 2000;11:521–536. doi: 10.1089/10430340050015716. [DOI] [PubMed] [Google Scholar]
- 22.Qing K, Khuntirat B, Mah C, Kube D M, Wang X S, Ponnazhagan S, Zhou S, Dwarki V J, Yoder M C, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: correlation of tyrosine phosphorylation of the cellular single-stranded D sequence-binding protein with transgene expression in human cells in vitro and murine tissues in vivo. J Virol. 1998;72:1593–1599. doi: 10.1128/jvi.72.2.1593-1599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutledge E A, Halbert C L, Russell D W. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samulski R J, Chang L S, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samulski R J, Chang L S, Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J Virol. 1987;61:3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson T A, Gould M N, Burkholder J K, Yang N S. Transient promoter activity in primary rat mammary epithelial cells evaluated using particle bombardment gene transfer. In Vitro Cell Dev Biol. 1993;29A:165–170. doi: 10.1007/BF02630949. [DOI] [PubMed] [Google Scholar]
- 28.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 29.Verma I M. A tumultuous year for gene therapy. Mol Ther. 2000;2:415–416. doi: 10.1006/mthe.2000.0213. [DOI] [PubMed] [Google Scholar]
- 30.Walsh K, Perlman H. Cell cycle exit upon myogenic differentiation. Curr Opin Genet Dev. 1997;7:597–602. doi: 10.1016/s0959-437x(97)80005-6. [DOI] [PubMed] [Google Scholar]
- 31.Walters R W, Duan D, Engelhardt J F, Welsh M J. Incorporation of adeno-associated virus in a calcium phosphate coprecipitate improves gene transfer to airway epithelia in vitro and in vivo. J Virol. 2000;74:535–540. doi: 10.1128/jvi.74.1.535-540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walters R W, Yi S M, Keshavjee S, Brown K E, Welsh M J, Chiorini J A, Zabner J. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem. 2001;21:21. doi: 10.1074/jbc.M101559200. [DOI] [PubMed] [Google Scholar]
- 33.Wang B, Li J, Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc Natl Acad Sci USA. 2000;97:13714–13719. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward W W, Bokman S H. Reversible denaturation of Aequorea green-fluorescent protein: physical separation and characterization of the renatured protein. Biochemistry. 1982;21:4535–4540. doi: 10.1021/bi00262a003. [DOI] [PubMed] [Google Scholar]
- 35.Wu P, Xiao W, Conlon T, Hughes J, Agbandje-McKenna M, Ferkol T, Flotte T, Muzyczka N. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. J Virol. 2000;74:8635–8647. doi: 10.1128/jvi.74.18.8635-8647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao W, Chirmule N, Berta S C, McCullough B, Gao G, Wilson J M. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao X, Li J, Samulski R J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaffe D, Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation. 1977;7:159–166. doi: 10.1111/j.1432-0436.1977.tb01507.x. [DOI] [PubMed] [Google Scholar]
- 39.Yan Z, Zhang Y, Duan D, Engelhardt J F. trans-splicing vectors expand the utility of adeno-associated virus for gene therapy. Proc Natl Acad Sci USA. 2000;97:6716–6721. doi: 10.1073/pnas.97.12.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zabner J, Seiler M, Walters R, Kotin R M, Fulgeras W, Davidson B L, Chiorini J A. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J Virol. 2000;74:3852–3858. doi: 10.1128/jvi.74.8.3852-3858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]