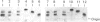Abstract
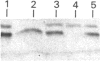
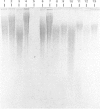
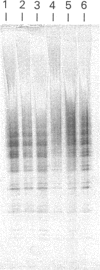
Amyloid fibrils were isolated by extraction in water from the livers and spleens of four patients who had died of monoclonal, light-chain (AL)-type, systemic amyloidosis and one with reactive systemic, amyloid A protein (AA)-type amyloidosis. Each fibril preparation contained 1-2% by weight of glycosaminoglycan (GAG) which was tightly associated with the fibrils and not just co-isolated from the tissues with them. After exhaustive digestion of the fibrils with papain and Pronase, the GAGs were specifically precipitated with cetylpyridinium chloride and were identified by cellulose acetate electrophoresis and selective susceptibility to specific glycosidases. All the preparations contained approximately equal amounts of heparan sulphate and dermatan sulphate. There was no evidence for the presence of chondroitin sulphate or other GAGs. Fine structural analysis by oligosaccharide mapping in gradient polyacrylamide gels, following partial digestion with specific glycosidases, showed very similar structures among the heparan sulphates and the dermatan sulphates, respectively. GAGs were also extracted by solubilizing amyloid fibrils in 4 M-guanidinium chloride followed by CsCl density-gradient ultracentrifugation. Although a minor proportion of the GAG material obtained in this way was apparently in the form of proteoglycan molecules, most of it was free GAG chains. The presence in amyloid fibrils of different types, in different organs and from different patients of particular GAG classes with similar structures supports the view that these molecules may be of pathogenic significance.
Full text
PDF
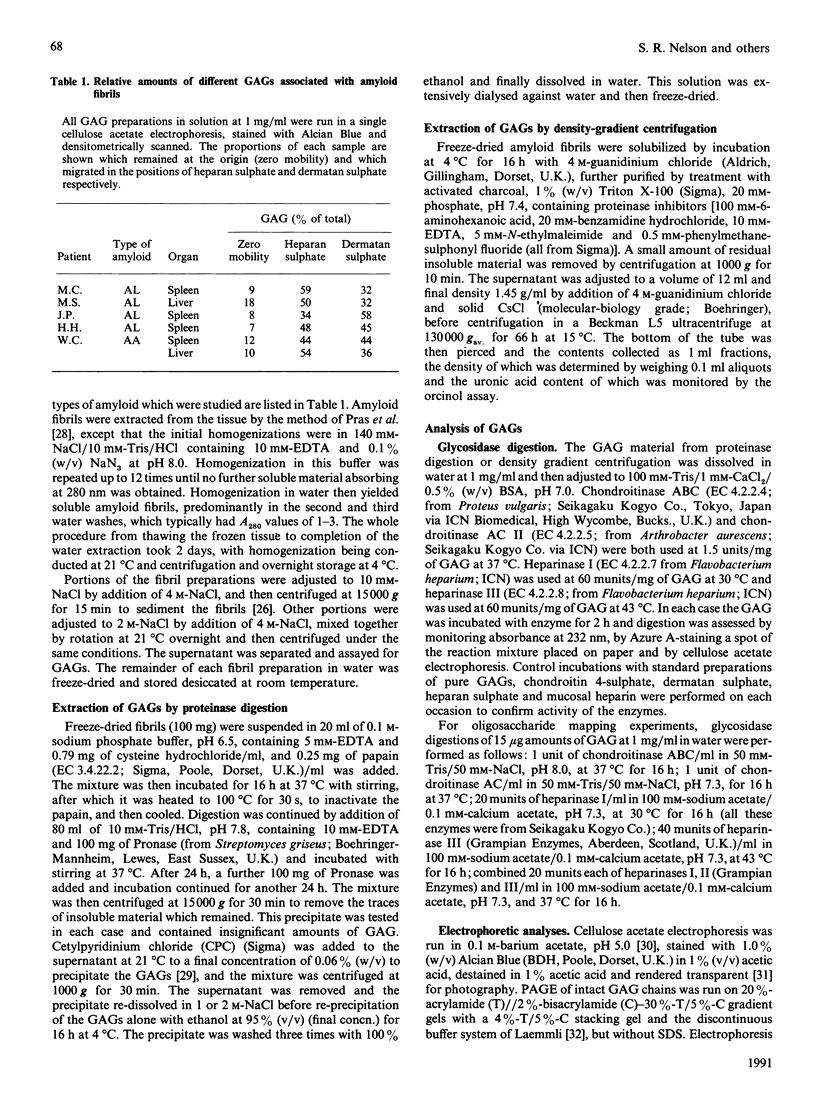

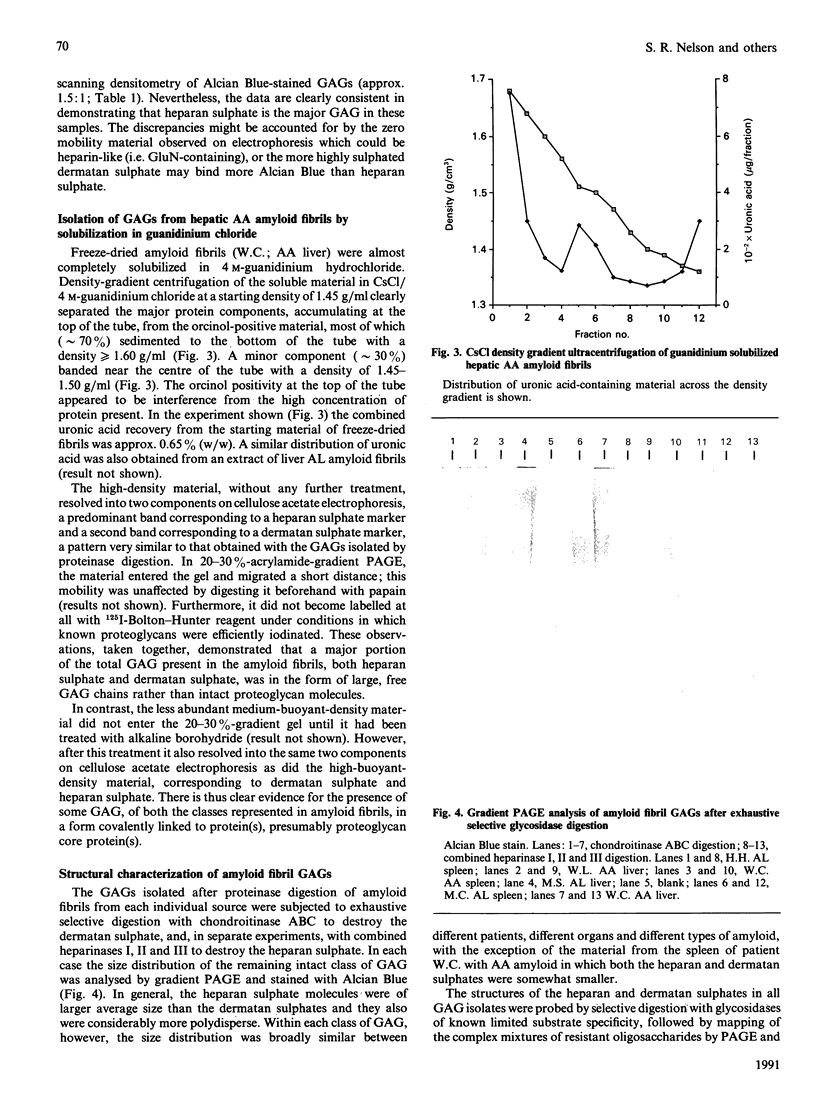



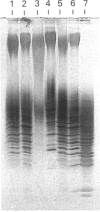
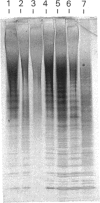
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitter T., Muir H. Mucopolysaccharides of whole human spleens in generalized amyloidosis. J Clin Invest. 1966 Jun;45(6):963–975. doi: 10.1172/JCI105412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen M., Weinstein H. G., Andersen M., Veis A. Microanalysis and characterization of acidic glycosaminoglycans in human tissues. Anal Biochem. 1970 May;35(1):146–159. doi: 10.1016/0003-2697(70)90020-5. [DOI] [PubMed] [Google Scholar]
- CESSI C., SERAFINI CESSI F. [Distribution of radiactive sulfate during the development of experimental myloidosis]. Boll Soc Ital Biol Sper. 1959 Dec 31;35:1785–1789. [PubMed] [Google Scholar]
- CESSI C., SERAFINICESSI F. RICERCHE SULL'AMILOIDE. III. L'AMILOIDOSI SPERIMENTALE DA POLIACIDI. Sperimentale. 1963 Mar-Apr;113:72–86. [PubMed] [Google Scholar]
- Cappelletti R., Del Rosso M., Chiarugi V. P. A new electrophoretic method for the complete separation of all known animal glycosaminoglycans in a monodimensional run. Anal Biochem. 1979 Nov 1;99(2):311–315. doi: 10.1016/s0003-2697(79)80012-3. [DOI] [PubMed] [Google Scholar]
- Dalferes E. R., Jr, Radhakrishnamurthy B., Berenson G. S. Acid mucopolysaccharides of amyloid tissue. Arch Biochem Biophys. 1967 Feb;118(2):284–291. doi: 10.1016/0003-9861(67)90350-5. [DOI] [PubMed] [Google Scholar]
- Dalferes E. R., Jr, Radhakrishnamurthy B., Berenson G. S. Glycosaminoglycans in experimental amyloidosis. Proc Soc Exp Biol Med. 1968 Mar;127(3):925–929. doi: 10.3181/00379727-127-32836. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T. The extended family of proteoglycans: social residents of the pericellular zone. Curr Opin Cell Biol. 1989 Dec;1(6):1201–1218. doi: 10.1016/s0955-0674(89)80072-9. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Hamazaki H. Ca2+-mediated association of human serum amyloid P component with heparan sulfate and dermatan sulfate. J Biol Chem. 1987 Feb 5;262(4):1456–1460. [PubMed] [Google Scholar]
- Johnson E. A. Characterisation and separation of sulphated glycosaminoglycuronans. Pharmacol Res Commun. 1982 Apr;14(4):289–320. doi: 10.1016/s0031-6989(82)80100-8. [DOI] [PubMed] [Google Scholar]
- KENNEDY J. S. Sulphur-35 in experimental amyloidosis. J Pathol Bacteriol. 1962 Jan;83:165–181. doi: 10.1002/path.1700830120. [DOI] [PubMed] [Google Scholar]
- Kolset S. O. Proteoglycans in normal and neoplastic monocytes. Exp Cell Res. 1987 Feb;168(2):318–324. doi: 10.1016/0014-4827(87)90004-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linhardt R. J., Turnbull J. E., Wang H. M., Loganathan D., Gallagher J. T. Examination of the substrate specificity of heparin and heparan sulfate lyases. Biochemistry. 1990 Mar 13;29(10):2611–2617. doi: 10.1021/bi00462a026. [DOI] [PubMed] [Google Scholar]
- Linker A., Carney H. C. Presence and role of glycosaminoglycans in amyloidosis. Lab Invest. 1987 Sep;57(3):297–305. [PubMed] [Google Scholar]
- Lyon M., Gallagher J. T. A general method for the detection and mapping of submicrogram quantities of glycosaminoglycan oligosaccharides on polyacrylamide gels by sequential staining with azure A and ammoniacal silver. Anal Biochem. 1990 Feb 15;185(1):63–70. doi: 10.1016/0003-2697(90)90255-8. [DOI] [PubMed] [Google Scholar]
- Magnus J. H., Husby G., Kolset S. O. Presence of glycosaminoglycans in purified AA type amyloid fibrils associated with juvenile rheumatoid arthritis. Ann Rheum Dis. 1989 Mar;48(3):215–219. doi: 10.1136/ard.48.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratil J. D., Murgia E., Walton H. F. Ligand-exchange chromatography of amino sugars. Anal Chem. 1975 Jan;47(1):122–125. doi: 10.1021/ac60351a003. [DOI] [PubMed] [Google Scholar]
- Norling B., Westermark G. T., Westermark P. Immunohistochemical identification of heparan sulphate proteoglycan in secondary systemic amyloidosis. Clin Exp Immunol. 1988 Aug;73(2):333–337. [PMC free article] [PubMed] [Google Scholar]
- Ohishi H., Skinner M., Sato-Araki N., Okuyama T., Gejyo F., Kimura A., Cohen A. S., Schmid K. Glycosaminoglycans of the hemodialysis-associated carpal synovial amyloid and of amyloid-rich tissues and fibrils of heart, liver, and spleen. Clin Chem. 1990 Jan;36(1):88–91. [PubMed] [Google Scholar]
- Pennock C. A. Association of acid mucopolysaccharides with isolated amyloid fibrils. Nature. 1968 Feb 24;217(5130):753–754. doi: 10.1038/217753a0. [DOI] [PubMed] [Google Scholar]
- Pennock C. A., Burns J., Massarella G. Histochemical investigation of acid mucosubstances in secondary amyloidosis. J Clin Pathol. 1968 Sep;21(5):578–581. doi: 10.1136/jcp.21.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter L. S., Chui H. C., Saperia D., Athanikar J. Microangiopathy and the colocalization of heparan sulfate proteoglycan with amyloid in senile plaques of Alzheimer's disease. Brain Res. 1990 Jan 29;508(1):13–19. doi: 10.1016/0006-8993(90)91111-s. [DOI] [PubMed] [Google Scholar]
- Pollak A., Coradello H., Latzka U., Lischka A., Lubec G. Wechselwirkungen zwischen Amyloid P und Bindegewebsproteinen. Wien Klin Wochenschr. 1982 May 28;94(11):291–293. [PubMed] [Google Scholar]
- Pras M., Nevo Z., Schubert M., Rotman J., Matalon R. The significance of mucopolysaccharides in amyloid. J Histochem Cytochem. 1971 Jul;19(7):443–448. doi: 10.1177/19.7.443. [DOI] [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989 Aug 15;264(23):13369–13372. [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- SERAFINICESSI F., CESSI C. RICERCHE SULL'AMILOIDE. IV. ISOLAMENTO DI UNA MUCOPROTEINA SOLFORATA DALL'AMILOIDE DA CASEINA DEL TOPO. Sperimentale. 1963 Mar-Apr;113:87–92. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Kisilevsky R., Stephens C., Anastassiades T. Characterization of tissue and plasma glycosaminoglycans during experimental AA amyloidosis and acute inflammation. Qualitative and quantitative analysis. Lab Invest. 1987 Jun;56(6):665–675. [PubMed] [Google Scholar]
- Snow A. D., Kisilevsky R. Temporal relationship between glycosaminoglycan accumulation and amyloid deposition during experimental amyloidosis. A histochemical study. Lab Invest. 1985 Jul;53(1):37–44. [PubMed] [Google Scholar]
- Snow A. D., Kisilevsky R., Willmer J., Prusiner S. B., DeArmond S. J. Sulfated glycosaminoglycans in amyloid plaques of prion diseases. Acta Neuropathol. 1989;77(4):337–342. doi: 10.1007/BF00687367. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Wight T. N. Proteoglycans in the pathogenesis of Alzheimer's disease and other amyloidoses. Neurobiol Aging. 1989 Sep-Oct;10(5):481–497. doi: 10.1016/0197-4580(89)90108-5. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Willmer J., Kisilevsky R. A close ultrastructural relationship between sulfated proteoglycans and AA amyloid fibrils. Lab Invest. 1987 Dec;57(6):687–698. [PubMed] [Google Scholar]
- Snow A. D., Willmer J., Kisilevsky R. Sulfated glycosaminoglycans: a common constituent of all amyloids? Lab Invest. 1987 Jan;56(1):120–123. [PubMed] [Google Scholar]
- Staprans I., Felts J. M. Isolation and characterization of glycosaminoglycans in human plasma. J Clin Invest. 1985 Nov;76(5):1984–1991. doi: 10.1172/JCI112198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward W. P., Christmas S. E., Lyon M., Gallagher J. T. The synthesis of proteoglycans by human T lymphocytes. Biochim Biophys Acta. 1990 May 22;1052(3):416–425. doi: 10.1016/0167-4889(90)90151-3. [DOI] [PubMed] [Google Scholar]
- Young I. D., Willmer J. P., Kisilevsky R. The ultrastructural localization of sulfated proteoglycans is identical in the amyloids of Alzheimer's disease and AA, AL, senile cardiac and medullary carcinoma-associated amyloidosis. Acta Neuropathol. 1989;78(2):202–209. doi: 10.1007/BF00688210. [DOI] [PubMed] [Google Scholar]