Abstract
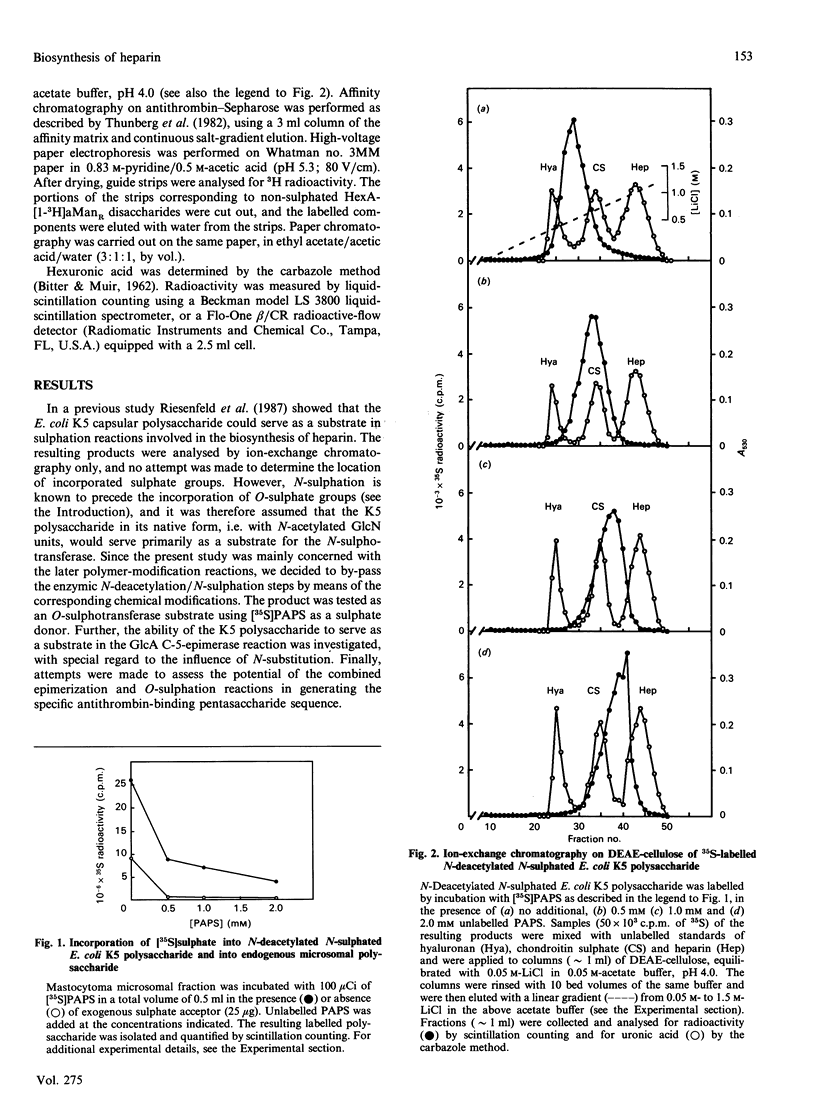
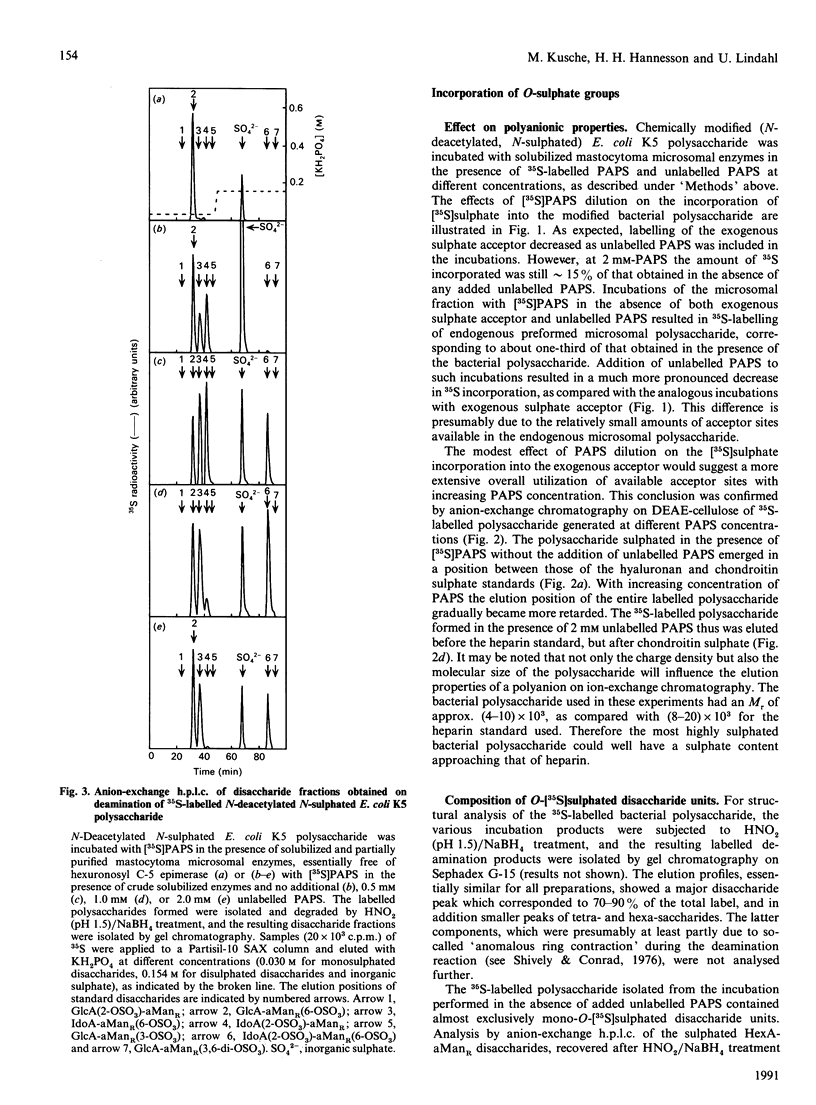
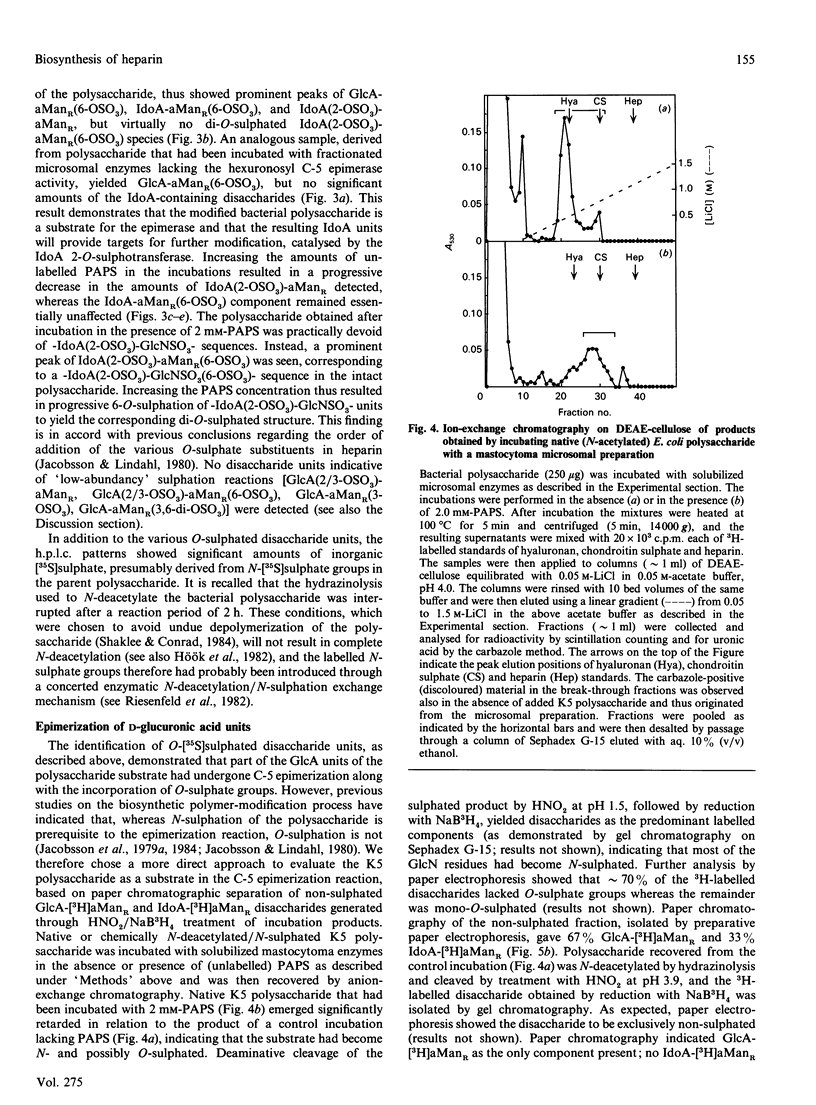
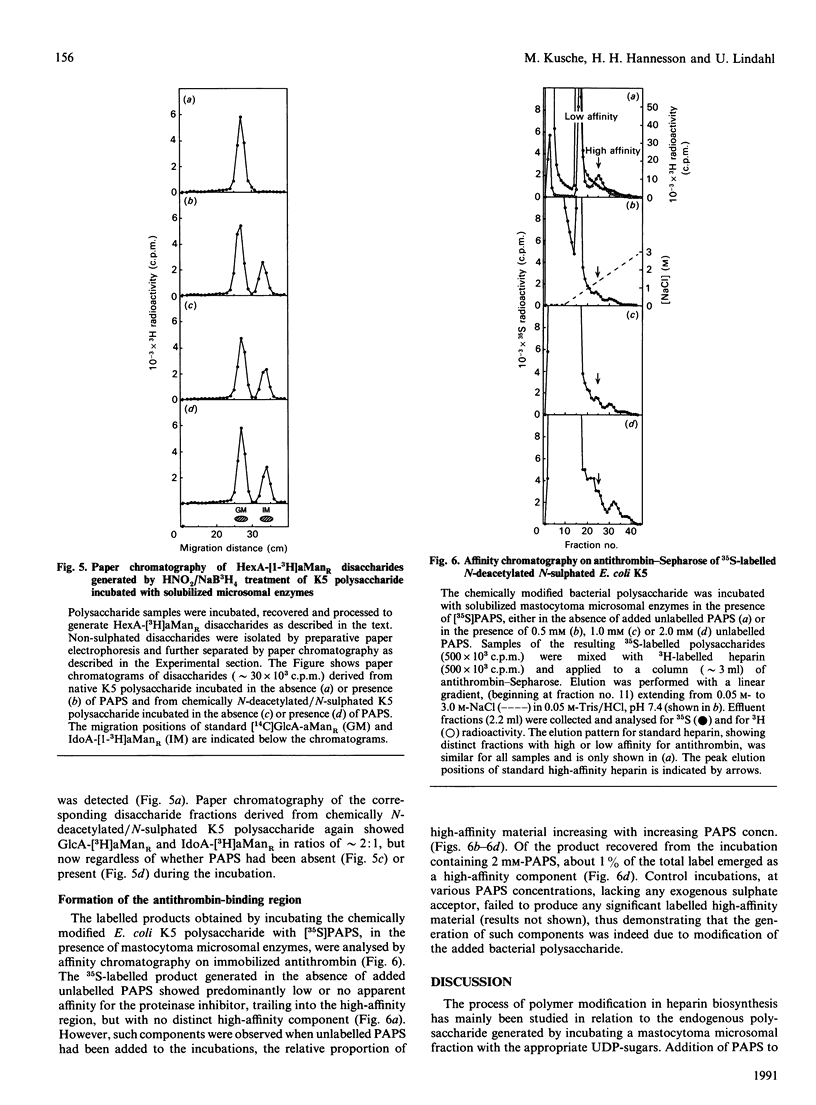
A capsular polysaccharide from Escherichia coli K5 was previously found to have the same structure, [-(4)beta GlcA(1)----(4)alpha GlcNAc(1)-]n, as that of the non-sulphated precursor polysaccharide in heparin biosynthesis [Vann, Schmidt, Jann & Jann (1981) Eur. J. Biochem. 116, 359-364]. The K5 polysaccharide was N-deacetylated (by hydrazinolysis) and N-sulphated, and was then incubated with detergent-solubilized enzymes from a heparin-producing mouse mastocytoma, in the presence of adenosine 3'-phosphate 5'-phospho[35S] sulphate ([35S]PAPS). Structural analysis of the resulting 35S-labelled polysaccharide revealed the formation of all the major disaccharide units found in heparin. The identification of 2-O-[35S]sulphated IdoA (L-iduronic acid) as well as 6-O-[35S]sulphated GlcNSO3 units demonstrated that the modified K5 polysaccharide served as a substrate in the hexuronosyl C-5-epimerase and the major O-sulphotransferase reactions involved in the biosynthesis of heparin. The GlcA units of the native (N-acetylated) E. coli polysaccharide were attacked by the epimerase only when PAPS was present in the incubations, whereas those of the chemically N-sulphated polysaccharide were epimerized also in the absence of PAPS, in accord with the notion that N-sulphate groups are required for epimerization. With increasing concentrations of PAPS, the mono-O-sulphated disaccharide unit-IdoA(2-OSO3)-GlcNSO3- was progressively converted into the di-O-sulphated species -IdoA(2-OSO3)-GlcNSO3(6-OSO3)-. A small proportion of the 35S-labelled polysaccharide was found to bind with high affinity to the proteinase inhibitor antithrombin. This proportion increased with increasing concentration of PAPS up to a level corresponding to approximately 1-2% of the total incorporated 35S. The solubilized enzymes thus catalysed all the reactions required for the generation of functional antithrombin-binding sites.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atha D. H., Lormeau J. C., Petitou M., Rosenberg R. D., Choay J. Contribution of monosaccharide residues in heparin binding to antithrombin III. Biochemistry. 1985 Nov 5;24(23):6723–6729. doi: 10.1021/bi00344a063. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bienkowski M. J., Conrad H. E. Structural characterization of the oligosaccharides formed by depolymerization of heparin with nitrous acid. J Biol Chem. 1985 Jan 10;260(1):356–365. [PubMed] [Google Scholar]
- Bäckström G., Hök M., Lindahl U., Feingold D. S., Malmström A., Rodén L., Jacobsson I. Biosynthesis of heparin. Assay and properties of the microsomal uronosyl C-5 epimerase. J Biol Chem. 1979 Apr 25;254(8):2975–2982. [PubMed] [Google Scholar]
- Enerbäck L., Kolset S. O., Kusche M., Hjerpe A., Lindahl U. Glycosaminoglycans in rat mucosal mast cells. Biochem J. 1985 Apr 15;227(2):661–668. doi: 10.1042/bj2270661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURTH J., HAGEN P., HIRSCH E. I. Transplantable mastocytoma in the mouse containing histamine, heparin, 5-hydroxytryptamine. Proc Soc Exp Biol Med. 1957 Aug-Sep;95(4):824–828. doi: 10.3181/00379727-95-23375. [DOI] [PubMed] [Google Scholar]
- Fedarko N. S., Conrad H. E. A unique heparan sulfate in the nuclei of hepatocytes: structural changes with the growth state of the cells. J Cell Biol. 1986 Feb;102(2):587–599. doi: 10.1083/jcb.102.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hök M., Lindahl U., Hallén A., Bäckström G. Biosynthesis of heparin. Studies on the microsomal sulfation process. J Biol Chem. 1975 Aug 10;250(15):6065–6071. [PubMed] [Google Scholar]
- Hök M., Riesenfeld J., Lindahl U. N-[3H]Acetyl-labeling, a convenient method for radiolabeling of glycosaminoglycans. Anal Biochem. 1982 Jan 15;119(2):236–245. doi: 10.1016/0003-2697(82)90580-2. [DOI] [PubMed] [Google Scholar]
- Ishihara M., Fedarko N. S., Conrad H. E. Transport of heparan sulfate into the nuclei of hepatocytes. J Biol Chem. 1986 Oct 15;261(29):13575–13580. [PubMed] [Google Scholar]
- Jacobsson I., Hök M., Pettersson I., Lindahl U., Larm O., Wirén E., von Figura K. Identification of N-sulphated disaccharide units in heparin-like polysaccharides. Biochem J. 1979 Apr 1;179(1):77–87. doi: 10.1042/bj1790077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson I., Lindahl U. Biosynthesis of heparin. Concerted action of late polymer-modification reactions. J Biol Chem. 1980 Jun 10;255(11):5094–5100. [PubMed] [Google Scholar]
- Jacobsson I., Lindahl U., Jensen J. W., Rodén L., Prihar H., Feingold D. S. Biosynthesis of heparin. Substrate specificity of heparosan N-sulfate D-glucuronosyl 5-epimerase. J Biol Chem. 1984 Jan 25;259(2):1056–1063. [PubMed] [Google Scholar]
- Kusche M., Bäckström G., Riesenfeld J., Petitou M., Choay J., Lindahl U. Biosynthesis of heparin. O-sulfation of the antithrombin-binding region. J Biol Chem. 1988 Oct 25;263(30):15474–15484. [PubMed] [Google Scholar]
- Kusche M., Lindahl U. Biosynthesis of heparin. O-sulfation of D-glucuronic acid units. J Biol Chem. 1990 Sep 15;265(26):15403–15409. [PubMed] [Google Scholar]
- Kusche M., Torri G., Casu B., Lindahl U. Biosynthesis of heparin. Availability of glucosaminyl 3-O-sulfation sites. J Biol Chem. 1990 May 5;265(13):7292–7300. [PubMed] [Google Scholar]
- LEVY L., PETRACEK F. J. Chemical and pharmacological studies on N-resulfated heparin. Proc Soc Exp Biol Med. 1962 Apr;109:901–905. doi: 10.3181/00379727-109-27372. [DOI] [PubMed] [Google Scholar]
- Lidholt K., Riesenfeld J., Jacobsson K. G., Feingold D. S., Lindahl U. Biosynthesis of heparin. Modulation of polysaccharide chain length in a cell-free system. Biochem J. 1988 Sep 1;254(2):571–578. doi: 10.1042/bj2540571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Thunberg L., Bäckström G., Riesenfeld J., Nordling K., Björk I. Extension and structural variability of the antithrombin-binding sequence in heparin. J Biol Chem. 1984 Oct 25;259(20):12368–12376. [PubMed] [Google Scholar]
- Navia J. L., Riesenfeld J., Vann W. F., Lindahl U., Rodén L. Assay of N-acetylheparosan deacetylase with a capsular polysaccharide from Escherichia coli K5 as substrate. Anal Biochem. 1983 Nov;135(1):134–140. doi: 10.1016/0003-2697(83)90741-8. [DOI] [PubMed] [Google Scholar]
- Pejler G., Bäckström G., Lindahl U., Paulsson M., Dziadek M., Fujiwara S., Timpl R. Structure and affinity for antithrombin of heparan sulfate chains derived from basement membrane proteoglycans. J Biol Chem. 1987 Apr 15;262(11):5036–5043. [PubMed] [Google Scholar]
- Petitou M., Duchaussoy P., Lederman I., Choay J., Sinaÿ P. Binding of heparin to antithrombin III: a chemical proof of the critical role played by a 3-sulfated 2-amino-2-deoxy-D-glucose residue. Carbohydr Res. 1988 Aug 15;179:163–172. doi: 10.1016/0008-6215(88)84116-8. [DOI] [PubMed] [Google Scholar]
- Riesenfeld J., Hök M., Lindahl U. Biosynthesis of heparin. Concerted action of early polymer-modification reactions. J Biol Chem. 1982 Jan 10;257(1):421–425. [PubMed] [Google Scholar]
- Shaklee P. N., Conrad H. E. Hydrazinolysis of heparin and other glycosaminoglycans. Biochem J. 1984 Jan 1;217(1):187–197. doi: 10.1042/bj2170187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Nearest neighbor analysis of heparin: identification and quantitation of the products formed by selective depolymerization procedures. Biochemistry. 1976 Sep 7;15(18):3943–3950. doi: 10.1021/bi00663a006. [DOI] [PubMed] [Google Scholar]
- Thunberg L., Bäckström G., Lindahl U. Further characterization of the antithrombin-binding sequence in heparin. Carbohydr Res. 1982 Mar 1;100:393–410. doi: 10.1016/s0008-6215(00)81050-2. [DOI] [PubMed] [Google Scholar]
- Vann W. F., Schmidt M. A., Jann B., Jann K. The structure of the capsular polysaccharide (K5 antigen) of urinary-tract-infective Escherichia coli 010:K5:H4. A polymer similar to desulfo-heparin. Eur J Biochem. 1981 May 15;116(2):359–364. doi: 10.1111/j.1432-1033.1981.tb05343.x. [DOI] [PubMed] [Google Scholar]


