Abstract
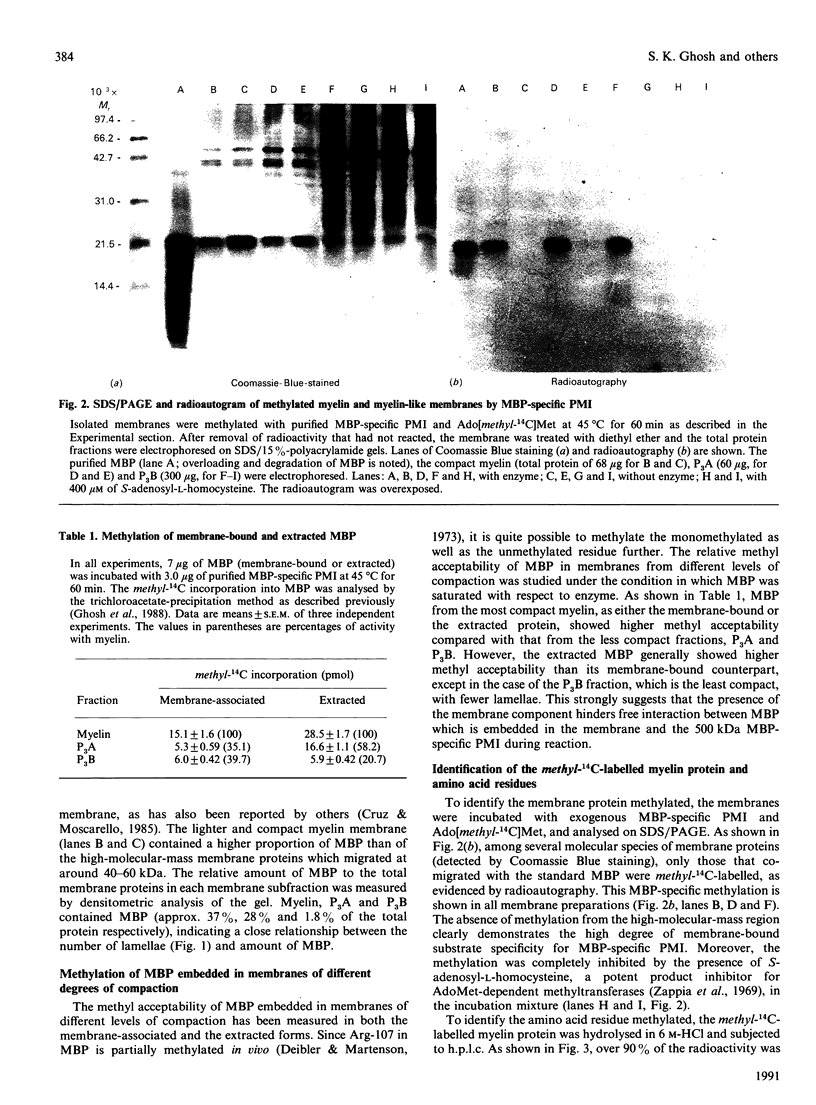
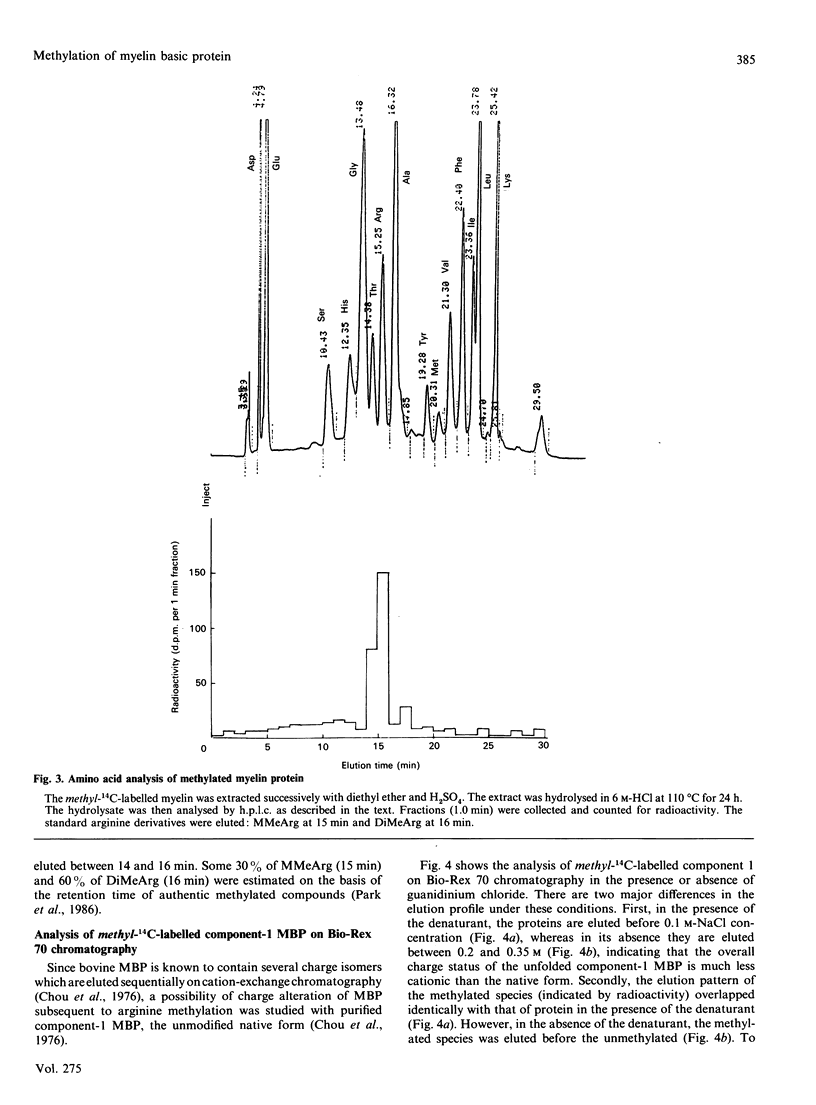
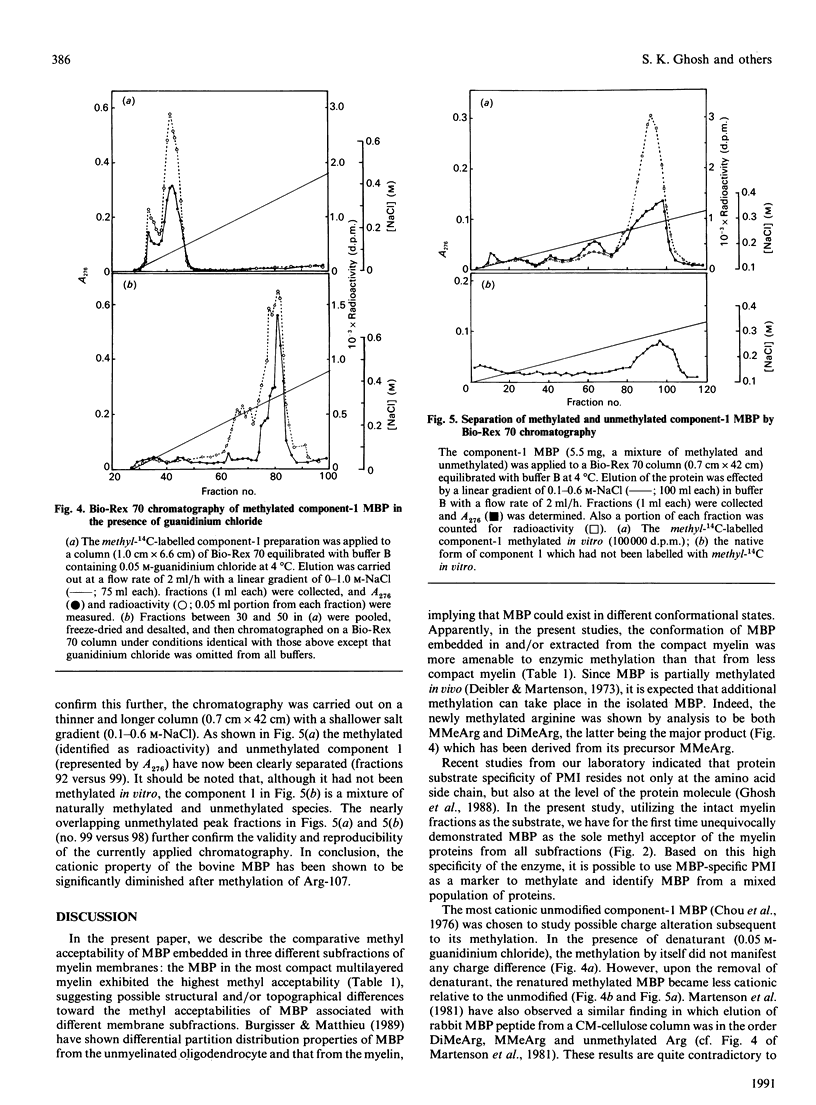
Myelin fractions with different degrees of compaction were isolated from bovine brain, and post-translational methylation of membrane-associated proteins was studied. When the purified myelin-basic-protein-specific protein methylase I and S-adenosyl-L-[methyl-14C]methionine were added exogenously, the most compact myelin fraction exhibited higher methyl-accepting activity than the less compact dense fractions. The methylated protein was identified as myelin basic protein (18.4 kDa) exclusively among the several myelin proteins from all membrane fractions, by SDS/PAGE/radioautography of methyl-14C-labelled membrane proteins. The methyl-14C-labelled amino acid residue in the basic protein was identified by h.p.l.c. as NG-methylarginine, indicating the high degree of specificity for the arginine residue as well as the myelin basic protein in the intact myelin membranes. The possibility of a charge alteration of myelin basic protein resulting from its arginine methylation was investigated by using the purified component 1 of myelin basic protein. The methylated component was shown to be less cationic than the unmethylated component by Bio-Rex 70 cation-exchange chromatography, since the former preceded the latter. However, in the presence of the denaturant (guanidinium chloride), the two species were co-eluted, indicating that the charge difference between methylated and unmethylated myelin basic protein can only be shown under the renatured condition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amur S. G., Shanker G., Pieringer R. A. Regulation of myelin basic protein (arginine) methyltransferase by thyroid hormone in myelinogenic cultures of cells dissociated from embryonic mouse brain. J Neurochem. 1984 Aug;43(2):494–498. doi: 10.1111/j.1471-4159.1984.tb00926.x. [DOI] [PubMed] [Google Scholar]
- Baldwin G. S., Carnegie P. R. Specific enzymic methylation of an arginine in the experimental allergic encephalomyelitis protein from human myelin. Science. 1971 Feb 12;171(3971):579–581. doi: 10.1126/science.171.3971.579. [DOI] [PubMed] [Google Scholar]
- Boggs J. M., Stamp D., Moscarello M. A. Interaction of myelin basic protein with dipalmitoylphosphatidylglycerol: dependence on the lipid phase and investigation of a metastable state. Biochemistry. 1981 Oct 13;20(21):6066–6072. doi: 10.1021/bi00524a023. [DOI] [PubMed] [Google Scholar]
- Brostoff S., Eylar E. H. Localization of methylated arginine in the A1 protein from myelin. Proc Natl Acad Sci U S A. 1971 Apr;68(4):765–769. doi: 10.1073/pnas.68.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürgisser P., Matthieu J. M. Phase separation of myelin proteins in triton X-114: differential behavior of myelin basic protein in purified myelin and in cultured oligodendrocytes. Dev Neurosci. 1989;11(3):179–187. doi: 10.1159/000111935. [DOI] [PubMed] [Google Scholar]
- Chanderkar L. P., Paik W. K., Kim S. Studies on myelin-basic-protein methylation during mouse brain development. Biochem J. 1986 Dec 1;240(2):471–479. doi: 10.1042/bj2400471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheifetz S., Moscarello M. A. Effect of bovine basic protein charge microheterogeneity on protein-induced aggregation of unilamellar vesicles containing a mixture of acidic and neutral phospholipids. Biochemistry. 1985 Apr 9;24(8):1909–1914. doi: 10.1021/bi00329a016. [DOI] [PubMed] [Google Scholar]
- Chou F. C., Chou C. H., Shapira R., Kibler R. F. Basis of microheterogeneity of myelin basic protein. J Biol Chem. 1976 May 10;251(9):2671–2679. [PubMed] [Google Scholar]
- Chou F. C., Chou C. H., Shapira R., Kibler R. F. Modifications of myelin basic protein which occur during its isolation. J Neurochem. 1977 May;28(5):1051–1059. doi: 10.1111/j.1471-4159.1977.tb10668.x. [DOI] [PubMed] [Google Scholar]
- Crang A. J., Jacobson W. The relationship of myelin basic protein (arginine) methyltransferase to myelination in mouse spinal cord. J Neurochem. 1982 Jul;39(1):244–247. doi: 10.1111/j.1471-4159.1982.tb04726.x. [DOI] [PubMed] [Google Scholar]
- Cruz T. F., Moscarello M. A. Characterization of myelin fractions from human brain white matter. J Neurochem. 1985 May;44(5):1411–1418. doi: 10.1111/j.1471-4159.1985.tb08777.x. [DOI] [PubMed] [Google Scholar]
- Deibler G. E., Martenson R. E. Determination of methylated basic amino acids with the amino acid analyzer. Application to total acid hydrolyzates of myelin basic proteins. J Biol Chem. 1973 Apr 10;248(7):2387–2391. [PubMed] [Google Scholar]
- Deibler G. E., Martenson R. E., Kies M. W. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2(2):139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- Deibler G. E., Martenson R. E., Kramer A. J., Kies M. W. The contribution of phosphorylation and loss of COOH-terminal arginine to the microheterogeneity of myelin basic protein. J Biol Chem. 1975 Oct 10;250(19):7931–7938. [PubMed] [Google Scholar]
- Edwards A. M., Ross N. W., Ulmer J. B., Braun P. E. Interaction of myelin basic protein and proteolipid protein. J Neurosci Res. 1989 Jan;22(1):97–102. doi: 10.1002/jnr.490220113. [DOI] [PubMed] [Google Scholar]
- Eylar E. H. Amino acid sequence of the basic protein of the myelin membrane. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1425–1431. doi: 10.1073/pnas.67.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser P. E., Deber C. M. Surface accessibility of 13C-labeled lysine residues in membrane-bound myelin basic protein. J Biol Chem. 1984 Jul 25;259(14):8689–8692. [PubMed] [Google Scholar]
- Ghosh S. K., Paik W. K., Kim S. Purification and molecular identification of two protein methylases I from calf brain. Myelin basic protein- and histone-specific enzyme. J Biol Chem. 1988 Dec 15;263(35):19024–19033. [PubMed] [Google Scholar]
- Kim S., Tuck M., Kim M., Campagnoni A. T., Paik W. K. Studies on myelin basic protein-specific protein methylase I in various dysmyelinating mutant mice. Biochem Biophys Res Commun. 1984 Sep 17;123(2):468–474. doi: 10.1016/0006-291x(84)90254-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowden J. A., Moscarello M. A., Morecki R. The isolation and characterization of an acid-soluble protein from myelin. Can J Biochem. 1966 May;44(5):567–577. doi: 10.1139/o66-068. [DOI] [PubMed] [Google Scholar]
- Martenson R. E., Law M. J., Deibler G. E. Identification of multiple in vivo phosphorylation sites in rabbit myelin basic protein. J Biol Chem. 1983 Jan 25;258(2):930–937. [PubMed] [Google Scholar]
- Martenson R. E., Lüthy V., Deibler G. E. Cleavage of rabbit myelin basic protein by pepsin. J Neurochem. 1981 Jan;36(1):58–68. doi: 10.1111/j.1471-4159.1981.tb02377.x. [DOI] [PubMed] [Google Scholar]
- Matthieu J. M., Quarles R. H., Brady R. O., Webster H. de F. Variation of proteins, enzyme markers and gangliosides in myelin subfractions. Biochim Biophys Acta. 1973 Dec 5;329(2):305–317. doi: 10.1016/0304-4165(73)90295-x. [DOI] [PubMed] [Google Scholar]
- Miyake M. Methylases of myelin basic protein and histone in rat brain. J Neurochem. 1975 May;24(5):909–915. doi: 10.1111/j.1471-4159.1975.tb03655.x. [DOI] [PubMed] [Google Scholar]
- Moscarello M. A., Brady G. W., Fein D. B., Wood D. D., Cruz T. F. The role of charge microheterogeneity of basic protein in the formation and maintenance of the multilayered structure of myelin: a possible role in multiple sclerosis. J Neurosci Res. 1986;15(1):87–99. doi: 10.1002/jnr.490150109. [DOI] [PubMed] [Google Scholar]
- Paik W. K., Kim S. Protein methylase I. Purification and properties of the enzyme. J Biol Chem. 1968 May 10;243(9):2108–2114. [PubMed] [Google Scholar]
- Park K. S., Frost B. F., Shin S., Park I. K., Kim S., Paik W. K. Effect of enzymatic methylation of yeast iso-1-cytochrome c on its isoelectric point. Arch Biochem Biophys. 1988 Nov 15;267(1):195–204. doi: 10.1016/0003-9861(88)90023-9. [DOI] [PubMed] [Google Scholar]
- Persaud R., Fraser P., Wood D. D., Moscarello M. A. The glycosylation of human myelin basic protein at threonines 95 and 98 occurs sequentially. Biochim Biophys Acta. 1988 Sep 8;966(3):357–361. doi: 10.1016/0304-4165(88)90085-2. [DOI] [PubMed] [Google Scholar]
- Schulz P., Cruz T. F., Moscarello M. A. Endogenous phosphorylation of basic protein in myelin of varying degrees of compaction. Biochemistry. 1988 Oct 4;27(20):7793–7799. doi: 10.1021/bi00420a031. [DOI] [PubMed] [Google Scholar]
- Smith R. Self-association of myelin basic protein: enhancement by detergents and lipids. Biochemistry. 1982 May 25;21(11):2697–2701. doi: 10.1021/bi00540a019. [DOI] [PubMed] [Google Scholar]
- Vadas E. B., Melançon P., Braun P. E., Galley W. C. Phosphorescence studies of the interaction of myelin basic protein with phosphatidylserine vesicles. Biochemistry. 1981 May 26;20(11):3110–3116. doi: 10.1021/bi00514a019. [DOI] [PubMed] [Google Scholar]
- Whitaker J. N., Snyder D. S. Studies of autoimmunity in multiple sclerosis. CRC Crit Rev Clin Neurobiol. 1984;1(1):45–82. [PubMed] [Google Scholar]
- Zappia V., Zydek-Cwick R., Schlenk F. The specificity of S-adenosylmethionine derivatives in methyl transfer reactions. J Biol Chem. 1969 Aug 25;244(16):4499–4509. [PubMed] [Google Scholar]
- Zimmerman A. W., Quarles R. H., de Webster H., Matthieu J. M., Brady R. O. Characterization and protein analysis of myelin subfractions in rat brain: developmental and regional comparisons. J Neurochem. 1975 Dec;25(6):749–757. doi: 10.1111/j.1471-4159.1975.tb04404.x. [DOI] [PubMed] [Google Scholar]