Abstract
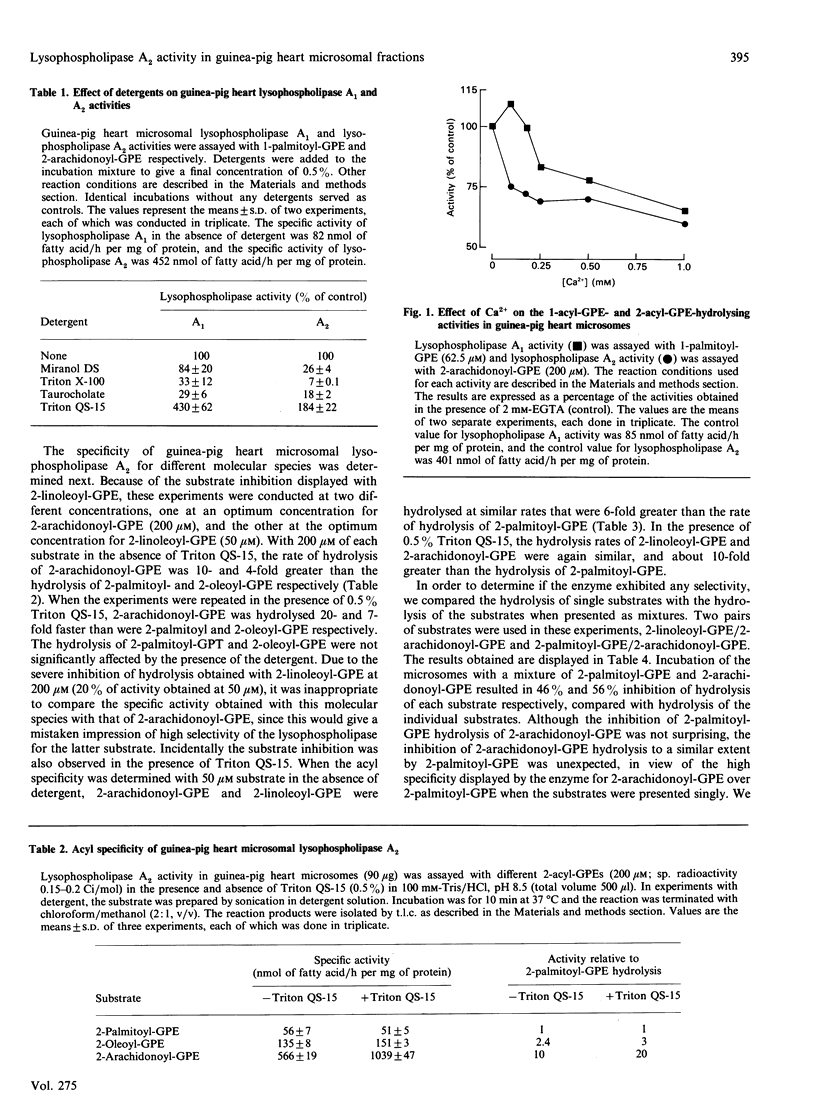
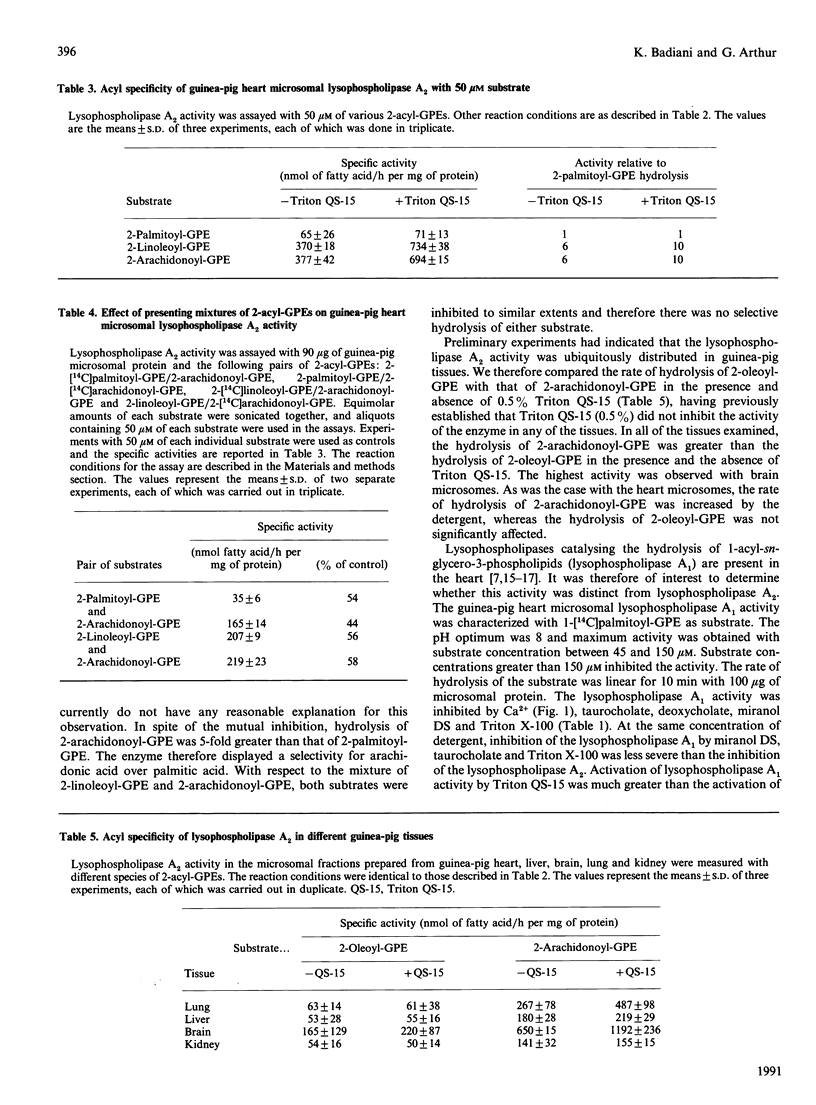
We have recently described a lysophospholipase A2 activity in guinea-pig heart microsomes that hydrolyses 2-acyl-sn-glycero-3-phosphocholine (2-acyl-GPC). The presence of a similar activity that hydrolyses 2-acyl-sn-glycero-3-phosphoethanolamine (2-acyl-GPE) was not known. In this study, a lysophospholipase A2 activity in guinea-pig heart microsomes that hydrolyses 2-acyl-GPE has been characterized. The enzyme did not require Ca2+ for activity and exhibited a high specificity for 2-arachidonoyl-GPE and 2-linoleoyl-GPE over 2-oleoyl-GPE and 2-palmitoyl-GPE. The specificity for these unsaturated substrates was observed in the presence and absence of detergents. Selective hydrolysis of 2-arachidonoyl-GPE over 2-palmitoyl-GPE was observed when equimolar quantities of the two substrates were incubated with the enzyme. There was no preferential hydrolysis of either 2-linoleoyl- or 2-arachidonoyl-GPE when presented individually or as a mixture. Significant differences in the characteristics of 2-acyl-GPE-hydrolysing and 2-acyl-GPC-hydrolysing activities included differences in their optimum pH, the effect of Ca2+ and their acyl specificities. Taken together, these results suggest that the two activities are catalysed by different enzymes. 2-Acyl-GPE lysophospholipase activity with a preference for 2-arachidonoyl-GPE over 2-oleoyl-GPE was observed in guinea-pig brain, liver, kidney and lung microsomes. Lysophospholipase A1 activity that catalyses the hydrolysis of 1-acyl-GPE was also present in guinea-pig heart microsomes and had different characteristics from the 2-acyl-GPE-hydrolysing activity, including a preference for saturated over unsaturated substrates. The 2-acyl-GPE lysophospholipase A2 activity appeared to be distinct from Ca(2+)-independent phospholipase A2. The characteristics of the 2-acyl-GPE lysophospholipase A2 suggest it could play a role in the selective release of arachidonic and linoleic acids for further metabolism in cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur G. Acylation of 2-acyl-glycerophosphocholine in guinea-pig heart microsomal fractions. Biochem J. 1989 Jul 15;261(2):575–580. doi: 10.1042/bj2610575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur G. Lysophospholipase A2 activity in guinea-pig heart microsomal fractions displaying high activities with 2-acylglycerophosphocholines with linoleic and arachidonic acids. Biochem J. 1989 Jul 15;261(2):581–586. doi: 10.1042/bj2610581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur G., Mock T., Zaborniak C., Choy P. C. The distribution and acyl composition of plasmalogens in guinea pig heart. Lipids. 1985 Oct;20(10):693–698. doi: 10.1007/BF02534389. [DOI] [PubMed] [Google Scholar]
- Comfurius P., Zwaal R. F. The enzymatic synthesis of phosphatidylserine and purification by CM-cellulose column chromatography. Biochim Biophys Acta. 1977 Jul 20;488(1):36–42. doi: 10.1016/0005-2760(77)90120-5. [DOI] [PubMed] [Google Scholar]
- Giffin M., Arthur G., Choy P. C., Man R. Y. Lysophosphatidylcholine metabolism and cardiac arrhythmias. Can J Physiol Pharmacol. 1988 Mar;66(3):185–189. doi: 10.1139/y88-032. [DOI] [PubMed] [Google Scholar]
- Gross R. W., Sobel B. E. Lysophosphatidylcholine metabolism in the rabbit heart. Characterization of metabolic pathways and partial purification of myocardial lysophospholipase-transacylase. J Biol Chem. 1982 Jun 25;257(12):6702–6708. [PubMed] [Google Scholar]
- HANAHAN D. J., RODBELL M., TURNER L. D. Enzymatic formation of monopalmitoleyl- and monopalmitoyllecithin (lysolecithins). J Biol Chem. 1954 Jan;206(1):431–441. [PubMed] [Google Scholar]
- Hazen S. L., Stuppy R. J., Gross R. W. Purification and characterization of canine myocardial cytosolic phospholipase A2. A calcium-independent phospholipase with absolute f1-2 regiospecificity for diradyl glycerophospholipids. J Biol Chem. 1990 Jun 25;265(18):10622–10630. [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lokesh B. R., Kinsella J. E. Intracellular calcium does not appear to be essential for arachidonic acid release from stimulated macrophages as shown by studies with Quin-2. Biochim Biophys Acta. 1985 Apr 22;845(1):101–108. doi: 10.1016/0167-4889(85)90060-6. [DOI] [PubMed] [Google Scholar]
- Nakashima S., Suganuma A., Matsui A., Hattori H., Sato M., Takenaka A., Nozawa Y. Primary role of calcium ions in arachidonic acid release from rat platelet membranes. Comparison with human platelet membranes. Biochem J. 1989 Apr 1;259(1):139–144. doi: 10.1042/bj2590139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock W. K., Rink T. J., Irvine R. F. Liberation of [3H]arachidonic acid and changes in cytosolic free calcium in fura-2-loaded human platelets stimulated by ionomycin and collagen. Biochem J. 1986 May 1;235(3):869–877. doi: 10.1042/bj2350869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz R. C., Lands W. E., Christie W. W., Holman R. T. Effects of ethylenic bond position upon acyltransferase activity with isomeric cis,cis-octadecadienoyl coenzyme A thiol esters. J Biol Chem. 1968 May 10;243(9):2241–2246. [PubMed] [Google Scholar]
- Severson D. L., Fletcher T. Regulation of lysophosphatidylcholine-metabolizing enzymes in isolated myocardial cells from rat heart. Can J Physiol Pharmacol. 1985 Aug;63(8):944–951. doi: 10.1139/y85-156. [DOI] [PubMed] [Google Scholar]
- Waite M., van Deenen L. L. Hydrolysis of phospholipids and glycerides by rat-liver preparations. Biochim Biophys Acta. 1967 Jun 6;137(3):498–517. doi: 10.1016/0005-2760(67)90131-2. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Intracellular phospholipases A. Biochim Biophys Acta. 1980 Sep 30;604(2):191–246. doi: 10.1016/0005-2736(80)90574-x. [DOI] [PubMed] [Google Scholar]


