Abstract
The biochemical and morphological consequences of procollagen prolyl 4-hydroxylase inhibition by pyridine-2,4-dicarboxylic acid (2,4-PDCA) and its diethyl ester (diethyl-2,4-PDC) were studied in chick-embryo calvaria, which predominantly synthesize type I collagen. Half-maximal inhibition of tissue hydroxyproline formation required 650 microM-2,4-PDCA, whereas the Ki with respect to chicken prolyl 4-hydroxylase in vitro was 2 microM. In contrast, half-maximal inhibition was caused by 10 microM-diethyl-2,4-PDC in the intact calvaria, although chicken prolyl 4-hydroxylase in vitro was not inhibited even at 1 mM. The collagenous material produced in the presence of diethyl-2,4-PDC showed an altered 'melting' profile and a lowering of the transition temperature by 10 degrees C, indicating misalignment and thermal instability of its triple-helical structure. Amount and electrophoretic mobility of procollagen type I chains were increased in a dose-dependent manner. The amounts of partially processed species and alpha-chains were decreased, without change in mobility. This marked effect on procollagen-collagen conversion in the intact calvaria suggests that the underhydroxylated collagenous material generated in the presence of diethyl-2,4-PDC is resistant to or acts as endogenous secondary inhibitor of type I procollagen N-proteinase. Electron microscopy of treated calvaria cells showed dilated rough endoplasmic reticulum and numerous phagolysosomes, indicating intracellular retention and lysosomal degradation of the newly synthesized underhydroxylated collagenous material. In summary, these results identify 2,4-PDCA and diethyl-2,4-PDC as the first prolyl 4-hydroxylase-directed inhibitor/proinhibitor pair that affects intra- and extra-cellular events during collagen formation.
Full text
PDF





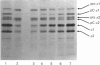
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. A., Prockop D. J. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun. 1973 May 1;52(1):115–120. doi: 10.1016/0006-291x(73)90961-3. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Prockop D. J. Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal Biochem. 1981 Jan 15;110(2):360–368. doi: 10.1016/0003-2697(81)90204-9. [DOI] [PubMed] [Google Scholar]
- Bächinger H. P., Bruckner P., Timpl R., Prockop D. J., Engel J. Folding mechanism of the triple helix in type-III collagen and type-III pN-collagen. Role of disulfide bridges and peptide bond isomerization. Eur J Biochem. 1980 May;106(2):619–632. doi: 10.1111/j.1432-1033.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- Chojkier M., Peterkofsky B., Bateman J. New method for determining the extent of proline hydroxylation by measuring changes in the ratio of [4-3H]:[14C]proline in collagenase digests. Anal Biochem. 1980 Nov 1;108(2):385–393. doi: 10.1016/0003-2697(80)90603-x. [DOI] [PubMed] [Google Scholar]
- Clark C. C., Richards C. F. Underhydroxylated minor cartilage collagen precursors cannot form stable triple helices. Biochem J. 1988 Feb 15;250(1):65–70. doi: 10.1042/bj2500065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski K. E., Sheats J. E., Prockop D. J. Iron-containing metallocenes as active site directed inhibitors of the proteinase that cleaves the NH2-terminal propeptides from type I procollagen. Biochemistry. 1986 Jul 29;25(15):4302–4309. doi: 10.1021/bi00363a019. [DOI] [PubMed] [Google Scholar]
- Eldridge C. F., Bunge R. P., Bunge M. B. Effects of cis-4-hydroxy-L-proline, and inhibitor of Schwann cell differentiation, on the secretion of collagenous and noncollagenous proteins by Schwann cells. Exp Cell Res. 1988 Feb;174(2):491–501. doi: 10.1016/0014-4827(88)90318-7. [DOI] [PubMed] [Google Scholar]
- Hanauske-Abel H. M., Günzler V. A stereochemical concept for the catalytic mechanism of prolylhydroxylase: applicability to classification and design of inhibitors. J Theor Biol. 1982 Jan 21;94(2):421–455. doi: 10.1016/0022-5193(82)90320-4. [DOI] [PubMed] [Google Scholar]
- Hata R., Kurata S., Shinkai H. Existence of malfunctioning pro alpha2(I) collagen genes in a patient with a pro alpha 2(I)-chain-defective variant of Ehlers-Danlos syndrome. Eur J Biochem. 1988 Jun 1;174(2):231–237. doi: 10.1111/j.1432-1033.1988.tb14087.x. [DOI] [PubMed] [Google Scholar]
- Hojima Y., van der Rest M., Prockop D. J. Type I procollagen carboxyl-terminal proteinase from chick embryo tendons. Purification and characterization. J Biol Chem. 1985 Dec 15;260(29):15996–16003. [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kedersha N. L., Berg R. A. An improved method for the purification of vertebrate prolyl hydroxylase by affinity chromatography. Coll Relat Res. 1981 Jul;1(4):345–353. doi: 10.1016/s0174-173x(81)80011-8. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Majamaa K. Synthesis of collagen: chemical regulation of post-translational events. Ciba Found Symp. 1985;114:34–64. doi: 10.1002/9780470720950.ch4. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Post-translational processing of procollagens. Ann N Y Acad Sci. 1985;460:187–201. doi: 10.1111/j.1749-6632.1985.tb51167.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Majamaa K., Günzler V., Hanauske-Abel H. M., Myllylä R., Kivirikko K. I. Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase. J Biol Chem. 1986 Jun 15;261(17):7819–7823. [PubMed] [Google Scholar]
- Majamaa K., Hanauske-Abel H. M., Günzler V., Kivirikko K. I. The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for 2-oxoglutarate decarboxylation in a ligand reaction at the enzyme-bound ferrous ion. Eur J Biochem. 1984 Jan 16;138(2):239–245. doi: 10.1111/j.1432-1033.1984.tb07907.x. [DOI] [PubMed] [Google Scholar]
- Majamaa K., Turpeenniemi-Hujanen T. M., Latipä P., Günzler V., Hanauske-Abel H. M., Hassinen I. E., Kivirikko K. I. Differences between collagen hydroxylases and 2-oxoglutarate dehydrogenase in their inhibition by structural analogues of 2-oxoglutarate. Biochem J. 1985 Jul 1;229(1):127–133. doi: 10.1042/bj2290127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa T., Tuderman L., Prockop D. J. Inhibitors of procollagen N-protease. Synthetic peptides with sequences similar to the cleavage site in the pro alpha 1(I) chain. Biochemistry. 1980 Jun 10;19(12):2646–2650. doi: 10.1021/bi00553a017. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., DiBlasio R. Modification of the tritium-release assays for prolyl and lysyl hydroxylases using Dowex-50 columns. Anal Biochem. 1975 May 26;66(1):279–286. doi: 10.1016/0003-2697(75)90747-2. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Majamaa K., Uitto J. Reduction of collagen production in keloid fibroblast cultures by ethyl-3,4-dihydroxybenzoate. Inhibition of prolyl hydroxylase activity as a mechanism of action. J Biol Chem. 1987 Jul 5;262(19):9397–9403. [PubMed] [Google Scholar]
- Sinkula A. A., Yalkowsky S. H. Rationale for design of biologically reversible drug derivatives: prodrugs. J Pharm Sci. 1975 Feb;64(2):181–210. doi: 10.1002/jps.2600640203. [DOI] [PubMed] [Google Scholar]
- Takehara K., Grotendorst G. R., Trojanowska M., Leroy E. C. Ascorbate effects on type I procollagen synthesis by human adult skin fibroblasts: different migration positions of type I procollagen chains on SDS polyacrylamide gel after incubation with ascorbate. Coll Relat Res. 1987 Feb;6(6):455–466. doi: 10.1016/s0174-173x(87)80045-6. [DOI] [PubMed] [Google Scholar]
- Tanzawa K., Berger J., Prockop D. J. Type I procollagen N-proteinase from whole chick embryos. Cleavage of a homotrimer of pro-alpha 1(I) chains and the requirement for procollagen with a triple-helical conformation. J Biol Chem. 1985 Jan 25;260(2):1120–1126. [PubMed] [Google Scholar]
- Tschank G., Hanauske-Abel H. M., Peterkofsky B. The effectiveness of inhibitors of soluble prolyl hydroxylase against the enzyme in the cisternae of isolated bone microsomes. Arch Biochem Biophys. 1988 Mar;261(2):312–323. doi: 10.1016/0003-9861(88)90346-3. [DOI] [PubMed] [Google Scholar]
- Tschank G., Raghunath M., Günzler V., Hanauske-Abel H. M. Pyridinedicarboxylates, the first mechanism-derived inhibitors for prolyl 4-hydroxylase, selectively suppress cellular hydroxyprolyl biosynthesis. Decrease in interstitial collagen and Clq secretion in cell culture. Biochem J. 1987 Dec 15;248(3):625–633. doi: 10.1042/bj2480625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuderman L., Kuutti E. R., Kivirikko K. I. An affinity-column procedure using poly(L-proline) for the purification of prolyl hydroxylase. Purification of the enzyme from chick embryos. Eur J Biochem. 1975 Mar 3;52(1):9–16. doi: 10.1111/j.1432-1033.1975.tb03967.x. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Prockop D. J. Procollagen N-proteinase. Properties of the enzyme purified from chick embryo tendons. Eur J Biochem. 1982 Jul;125(3):545–549. doi: 10.1111/j.1432-1033.1982.tb06716.x. [DOI] [PubMed] [Google Scholar]