Abstract
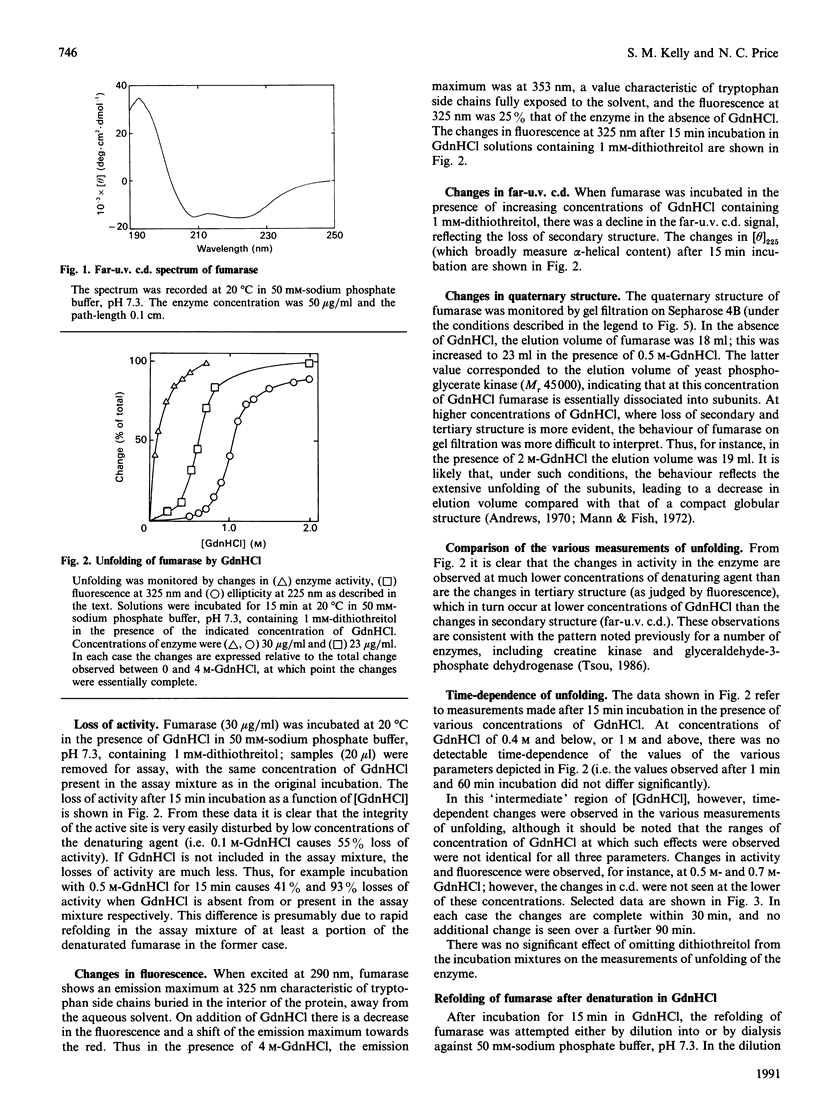
The unfolding of pig heart fumarase in solutions of guanidinium chloride (GdnHCl) has been examined. Loss of activity occurs at lower concentrations of GdnHCl than the structural changes detected by fluorescence or c.d. After denaturation, regain of activity can be observed provided that a reducing agent (dithiothreitol) is present and that the concentration of GdnHCl is lowered by dialysis rather than by dilution. The regain of secondary structure occurs with high efficiency even when little or no activity is recovered.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- Beeckmans S., Kanarek L. A new purification procedure for fumarase based of affinity chromatography. Isolation and characterization of pig-liver fumarase. Eur J Biochem. 1977 Sep;78(2):437–444. doi: 10.1111/j.1432-1033.1977.tb11756.x. [DOI] [PubMed] [Google Scholar]
- Beeckmans S., Kanarek L. Demonstration of physical interactions between consecutive enzymes of the citric acid cycle and of the aspartate-malate shuttle. A study involving fumarase, malate dehydrogenase, citrate synthesis and aspartate aminotransferase. Eur J Biochem. 1981 Jul;117(3):527–535. doi: 10.1111/j.1432-1033.1981.tb06369.x. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Lin L. N. Proline isomerization studied with proteolytic enzymes. Methods Enzymol. 1986;131:107–126. doi: 10.1016/0076-6879(86)31037-1. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989 Feb 16;337(6208):620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Protein folding. Biochem J. 1990 Aug 15;270(1):1–16. doi: 10.1042/bj2700001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J., Hemmingsen S. M. Molecular chaperones: proteins essential for the biogenesis of some macromolecular structures. Trends Biochem Sci. 1989 Aug;14(8):339–342. doi: 10.1016/0968-0004(89)90168-0. [DOI] [PubMed] [Google Scholar]
- Jaenicke R. Folding and association of proteins. Prog Biophys Mol Biol. 1987;49(2-3):117–237. doi: 10.1016/0079-6107(87)90011-3. [DOI] [PubMed] [Google Scholar]
- KANAREK L., HILL R. L. THE PREPARATION AND CHARACTERIZATION OF FUMARASE FROM SWINE HEART MUSCLE. J Biol Chem. 1964 Dec;239:4202–4206. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- London J., Skrzynia C., Goldberg M. E. Renaturation of Escherichia coli tryptophanase after exposure to 8 M urea. Evidence for the existence of nucleation centers. Eur J Biochem. 1974 Sep 1;47(2):409–415. doi: 10.1111/j.1432-1033.1974.tb03707.x. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Fish W. W. Protein polypeptide chain molecular weights by gel chromatography in guanidinium chloride. Methods Enzymol. 1972;26:28–42. doi: 10.1016/s0076-6879(72)26004-9. [DOI] [PubMed] [Google Scholar]
- Mitraki A., Betton J. M., Desmadril M., Yon J. M. Quasi-irreversibility in the unfolding-refolding transition of phosphoglycerate kinase induced by guanidine hydrochloride. Eur J Biochem. 1987 Feb 16;163(1):29–34. doi: 10.1111/j.1432-1033.1987.tb10732.x. [DOI] [PubMed] [Google Scholar]
- Nozaki Y. The preparation of guanidine hydrochloride. Methods Enzymol. 1972;26:43–50. doi: 10.1016/s0076-6879(72)26005-0. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Teipel J. W., Hill R. L. The subunit interactions of fumarase. J Biol Chem. 1971 Aug 10;246(15):4859–4865. [PubMed] [Google Scholar]
- Teipel J. W., Koshland D. E., Jr Kinetic aspects of conformational changes in proteins. I. Rate of regain of enzyme activity from denatured proteins. Biochemistry. 1971 Mar 2;10(5):792–798. doi: 10.1021/bi00781a011. [DOI] [PubMed] [Google Scholar]
- West S. M., Kelly S. M., Price N. C. The unfolding and attempted refolding of citrate synthase from pig heart. Biochim Biophys Acta. 1990 Mar 1;1037(3):332–336. doi: 10.1016/0167-4838(90)90034-d. [DOI] [PubMed] [Google Scholar]
- West S. M., Price N. C. The unfolding and attempted refolding of mitochondrial aspartate aminotransferase from pig heart. Biochem J. 1990 Jan 1;265(1):45–50. doi: 10.1042/bj2650045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. M., Price N. C. The unfolding and refolding of glutamate dehydrogenases from bovine liver, baker's yeast and Clostridium symbosium. Biochem J. 1988 Apr 1;251(1):135–139. doi: 10.1042/bj2510135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettlmeissl G., Rudolph R., Jaenicke R. Reconstitution of lactic dehydrogenase after acid dissociation. The yield of reactivation is determined by conformational rearrangements of the dissociated monomers. Eur J Biochem. 1981 Dec;121(1):169–175. doi: 10.1111/j.1432-1033.1981.tb06446.x. [DOI] [PubMed] [Google Scholar]


