Abstract
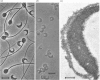
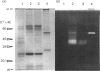
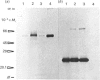
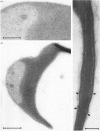
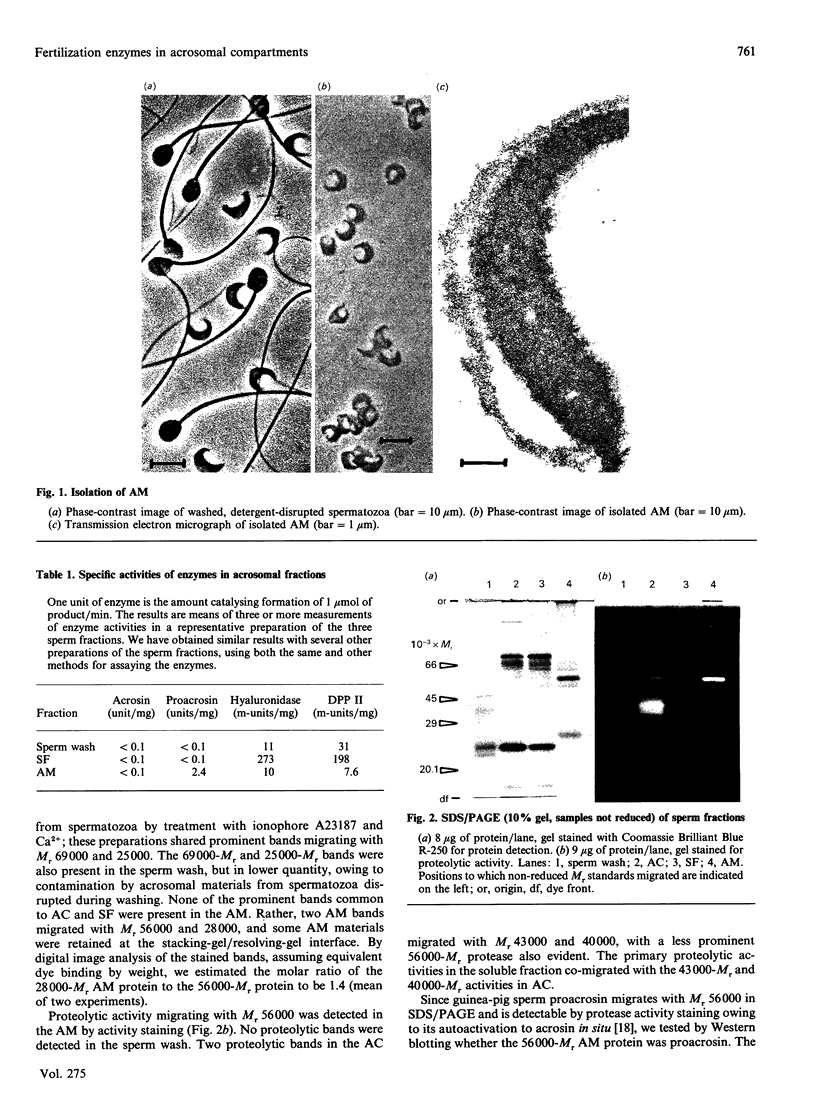
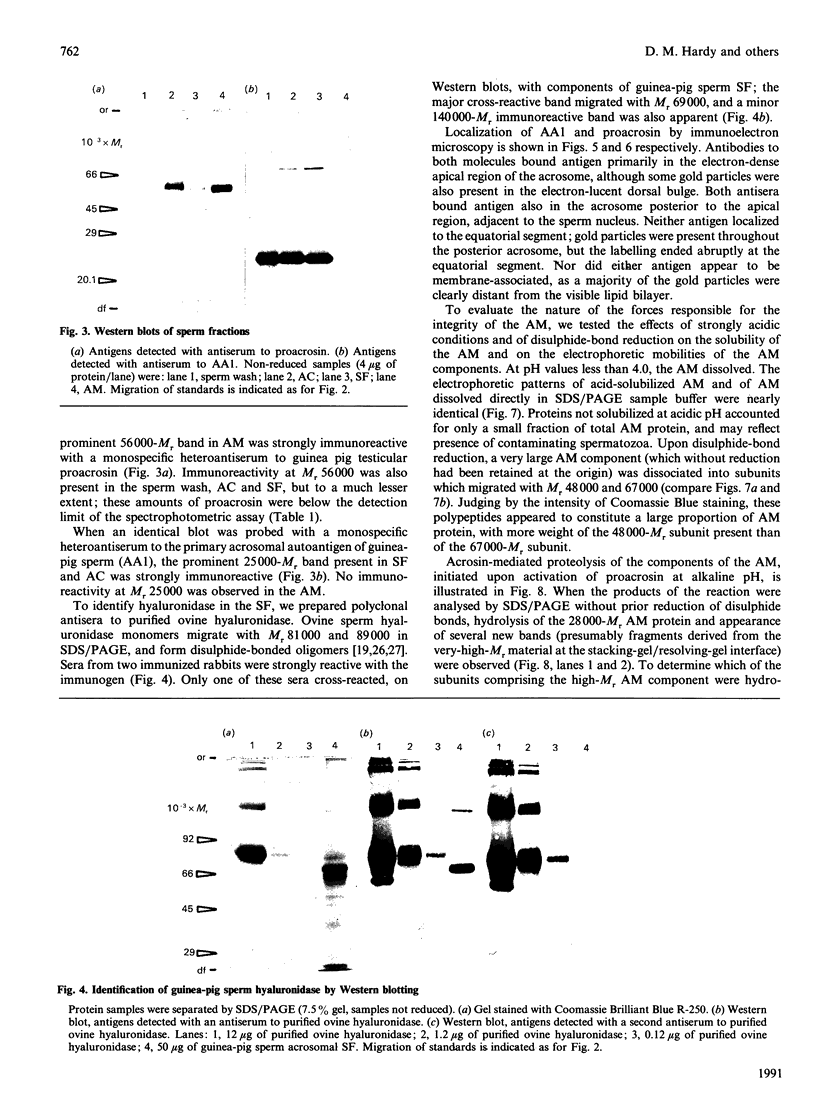
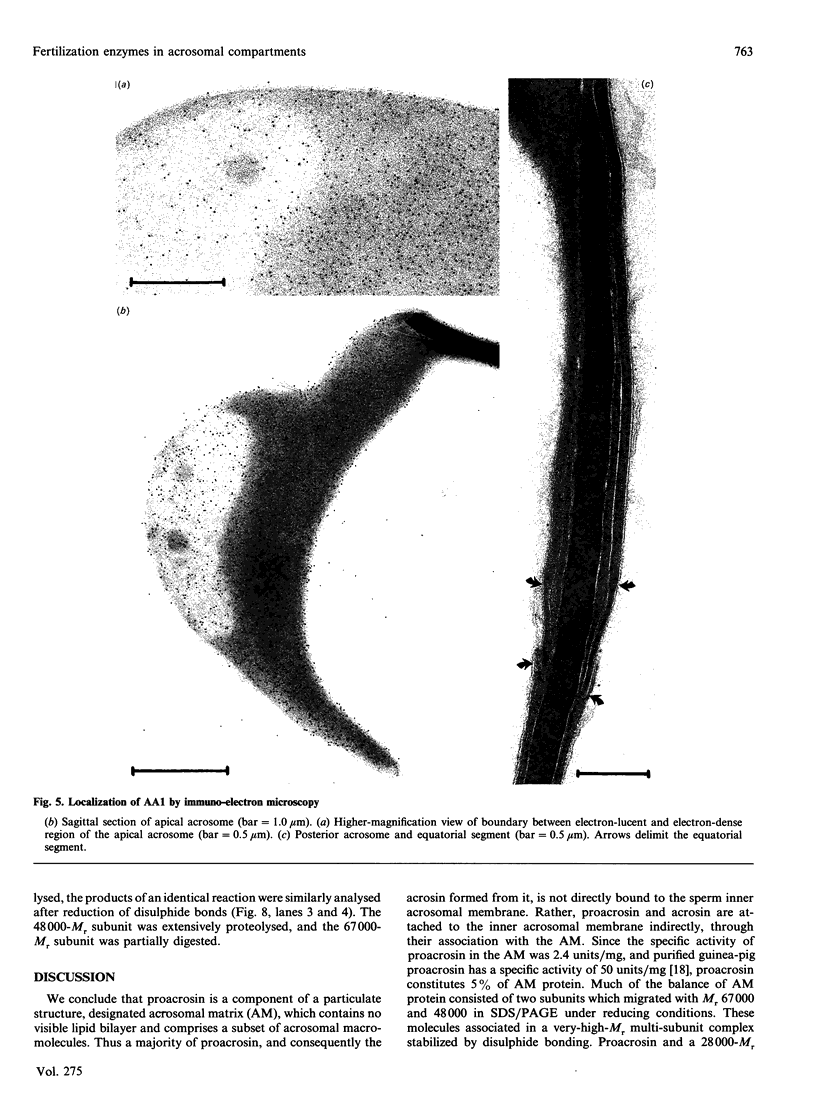
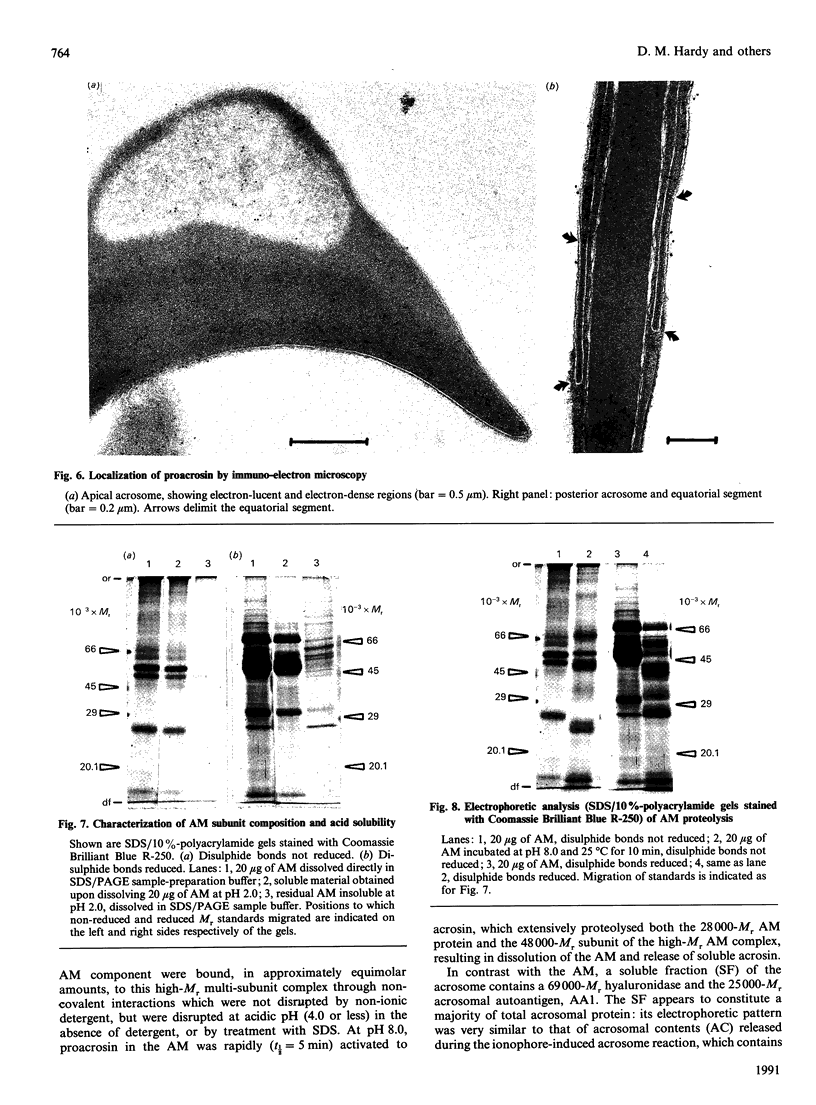
To study the organization of fertilization enzymes in the sperm acrosome, we isolated and characterized two physicochemically distinct acrosomal fractions of guinea-pig spermatozoa. A soluble fraction contained the 25,000-Mr acrosomal autoantigen, AA1, and most of the acrosomal hyaluronidase and dipeptidyl peptidase II activity. A particulate fraction, designated acrosomal matrix (AM), consisted of membraneless crescent-shaped structures, and contained most of the acrosomal proacrosin. The AM also contained a 28,000-Mr putative proacrosin-binding protein, and a very-high-Mr component which, on reduction, was dissociated into 48,000-Mr and 67,000-Mr subunits. Autoproteolytic dissolution of the AM correlated with proteolysis by acrosin of the 28,000-Mr and 48,000-Mr AM molecules. Components of both the AM and the soluble fraction were localized by immuno-electron microscopy to the electron-dense region of the guinea-pig sperm acrosome. We conclude that acrosomal molecules are segregated into soluble and matrix compartments. This segregation is a function of disulphide bonding and non-covalent interactions among the relatively few components of the AM. Association of acrosin with the AM may be the mechanism by which this enzyme's release from the spermatozoon during the acrosome reaction is delayed relative to the release of other acrosomal molecules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleil J. D., Greve J. M., Wassarman P. M. Identification of a secondary sperm receptor in the mouse egg zona pellucida: role in maintenance of binding of acrosome-reacted sperm to eggs. Dev Biol. 1988 Aug;128(2):376–385. doi: 10.1016/0012-1606(88)90299-0. [DOI] [PubMed] [Google Scholar]
- Brown C. R., Harrison R. A. The activation of proacrosin in spermatozoa from ram bull and boar. Biochim Biophys Acta. 1978 Sep 11;526(1):202–217. doi: 10.1016/0005-2744(78)90305-4. [DOI] [PubMed] [Google Scholar]
- Carroll E. J., Jr, Hedrick J. L. Hatching in the toad Xenopus laevis: morphological events and evidence for a hatching enzyme. Dev Biol. 1974 May;38(1):1–13. doi: 10.1016/0012-1606(74)90254-1. [DOI] [PubMed] [Google Scholar]
- DiCarlantonio G., Talbot P. Evidence for sequential deployment of secretory enzymes during the normal acrosome reaction of guinea pig sperm in vitro. Gamete Res. 1988 Dec;21(4):425–438. doi: 10.1002/mrd.1120210410. [DOI] [PubMed] [Google Scholar]
- Green D. P., Hockaday A. R. The histochemical localization of acrosin in guinea-pig sperm after the acrosome reaction. J Cell Sci. 1978 Aug;32:177–184. doi: 10.1242/jcs.32.1.177. [DOI] [PubMed] [Google Scholar]
- Green D. P. The activation of proteolysis in the acrosome reaction of guinea-pig sperm. J Cell Sci. 1978 Aug;32:153–164. doi: 10.1242/jcs.32.1.153. [DOI] [PubMed] [Google Scholar]
- Green D. P. The induction of the acrosome reaction in guinea-pig sperm by the divalent metal cation ionophore A23187. J Cell Sci. 1978 Aug;32:137–151. doi: 10.1242/jcs.32.1.137. [DOI] [PubMed] [Google Scholar]
- Hardy D. M., Huang T. T., Jr, Driscoll W. J., Tung K. K., Wild G. C. Purification and characterization of the primary acrosomal autoantigen of guinea pig epididymal spermatozoa. Biol Reprod. 1988 Mar;38(2):423–437. doi: 10.1095/biolreprod38.2.423. [DOI] [PubMed] [Google Scholar]
- Hardy D. M., Wild G. C., Tung K. S. Purification and initial characterization of proacrosins from guinea pig testes and epididymal spermatozoa. Biol Reprod. 1987 Aug;37(1):189–199. doi: 10.1095/biolreprod37.1.189. [DOI] [PubMed] [Google Scholar]
- Harrison R. A., Gaunt S. J. Multiple forms of ram and bull sperm hyaluronidase revealed by using monoclonal antibodies. J Reprod Fertil. 1988 Mar;82(2):777–785. doi: 10.1530/jrf.0.0820777. [DOI] [PubMed] [Google Scholar]
- Harrison R. A. Hyaluronidase in ram semen. Quantitative determination, and isolation of multiple forms. Biochem J. 1988 Jun 15;252(3):865–874. doi: 10.1042/bj2520865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. A. Preliminary characterization of the multiple forms of ram sperm hyaluronidase. Biochem J. 1988 Jun 15;252(3):875–882. doi: 10.1042/bj2520875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. A. The interaction of ram proacrosin and acrosin forms with antiserum raised against ram m beta-acrosin. J Reprod Immunol. 1982 Aug;4(4):231–249. doi: 10.1016/0165-0378(82)90029-8. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Wardrip N. J. On the macromolecular composition of the zona pellucida from porcine oocytes. Dev Biol. 1987 Jun;121(2):478–488. doi: 10.1016/0012-1606(87)90184-9. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Huang T. T., Fleming A. D., Yanagimachi R. Only acrosome-reacted spermatozoa can bind to and penetrate zona pellucida: a study using the guinea pig. J Exp Zool. 1981 Aug;217(2):287–290. doi: 10.1002/jez.1402170215. [DOI] [PubMed] [Google Scholar]
- Huang T. T., Jr, Hardy D., Yanagimachi H., Teuscher C., Tung K., Wild G., Yanagimachi R. pH and protease control of acrosomal content stasis and release during the guinea pig sperm acrosome reaction. Biol Reprod. 1985 Mar;32(2):451–462. doi: 10.1095/biolreprod32.2.451. [DOI] [PubMed] [Google Scholar]
- Hyatt H., Gwatkin R. B. Characterization of isolated acrosomal matrices from hamster spermatozoa. J Reprod Fertil. 1988 May;83(1):419–429. doi: 10.1530/jrf.0.0830419. [DOI] [PubMed] [Google Scholar]
- Jones R., Brown C. R. Identification of a zona-binding protein from boar spermatozoa as proacrosin. Exp Cell Res. 1987 Aug;171(2):503–508. doi: 10.1016/0014-4827(87)90182-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Müller-Esterl W., Fritz H. Interactions of boar acrosin with detergents. Hoppe Seylers Z Physiol Chem. 1980 Nov;361(11):1673–1682. doi: 10.1515/bchm2.1980.361.2.1673. [DOI] [PubMed] [Google Scholar]
- Noland T. D., Davis L. S., Olson G. E. Regulation of proacrosin conversion in isolated guinea pig sperm acrosomal apical segments. J Biol Chem. 1989 Aug 15;264(23):13586–13590. [PubMed] [Google Scholar]
- Olson G. E., Winfrey V. P., Davenport G. R. Characterization of matrix domains of the hamster acrosome. Biol Reprod. 1988 Dec;39(5):1145–1158. doi: 10.1095/biolreprod39.5.1145. [DOI] [PubMed] [Google Scholar]
- Parrish R. F., Polakoski K. L. An apparent high molecular weight form of boar proacrosin resulting from the presence of a protein that binds to proacrosin. Anal Biochem. 1978 Jun 15;87(1):108–113. doi: 10.1016/0003-2697(78)90574-2. [DOI] [PubMed] [Google Scholar]
- Parrish R. F., Polakoski K. L. Mammalian sperm proacrosin-acrosin system. Int J Biochem. 1979;10(5):391–395. doi: 10.1016/0020-711x(79)90061-2. [DOI] [PubMed] [Google Scholar]
- Parrish R. F., Straus W., Polakoski K. L., Dombrose F. A. Phospholipid vesicle stimulation of proacrosin activation. Proc Natl Acad Sci U S A. 1978 Jan;75(1):149–152. doi: 10.1073/pnas.75.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff P., Myles D. G., Bellvé A. R. Biochemical analysis of the released products of the mammalian acrosome reaction. Dev Biol. 1980 Dec;80(2):324–331. doi: 10.1016/0012-1606(80)90408-x. [DOI] [PubMed] [Google Scholar]
- SCHWERT G. W., TAKENAKA Y. A spectrophotometric determination of trypsin and chymotrypsin. Biochim Biophys Acta. 1955 Apr;16(4):570–575. doi: 10.1016/0006-3002(55)90280-8. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stojanoff A., Bourne H., Andrews A. G., Hyne R. V. Isolation of a stable apical segment of the guinea pig sperm acrosome. Gamete Res. 1987 Aug;17(4):321–332. doi: 10.1002/mrd.1120170405. [DOI] [PubMed] [Google Scholar]
- Storey B. T., Lee M. A., Muller C., Ward C. R., Wirtshafter D. G. Binding of mouse spermatozoa to the zonae pellucidae of mouse eggs in cumulus: evidence that the acrosomes remain substantially intact. Biol Reprod. 1984 Dec;31(5):1119–1128. doi: 10.1095/biolreprod31.5.1119. [DOI] [PubMed] [Google Scholar]
- Straus J. W., Parrish R. F., Polakoski K. L. Boar acrosin. Association of an endogenous membrane proteinase with phospholipid membranes. J Biol Chem. 1981 Jun 10;256(11):5662–5668. [PubMed] [Google Scholar]
- Straus J. W., Polakoski K. L. Association of proacrosin with phospholipid membranes. Biochem J. 1982 Mar 1;201(3):657–660. doi: 10.1042/bj2010657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P., Dicarlantonio G. Cytochemical localization of dipeptidyl peptidase II (DPP-II) in mature guinea pig sperm. J Histochem Cytochem. 1985 Nov;33(11):1169–1172. doi: 10.1177/33.11.4056380. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Application of cryoultramicrotomy to immunocytochemistry. J Microsc. 1986 Aug;143(Pt 2):139–149. doi: 10.1111/j.1365-2818.1986.tb02772.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töpfer-Petersen E., Henschen A. Acrosin shows zona and fucose binding, novel properties for a serine proteinase. FEBS Lett. 1987 Dec 21;226(1):38–42. doi: 10.1016/0014-5793(87)80546-x. [DOI] [PubMed] [Google Scholar]