Abstract
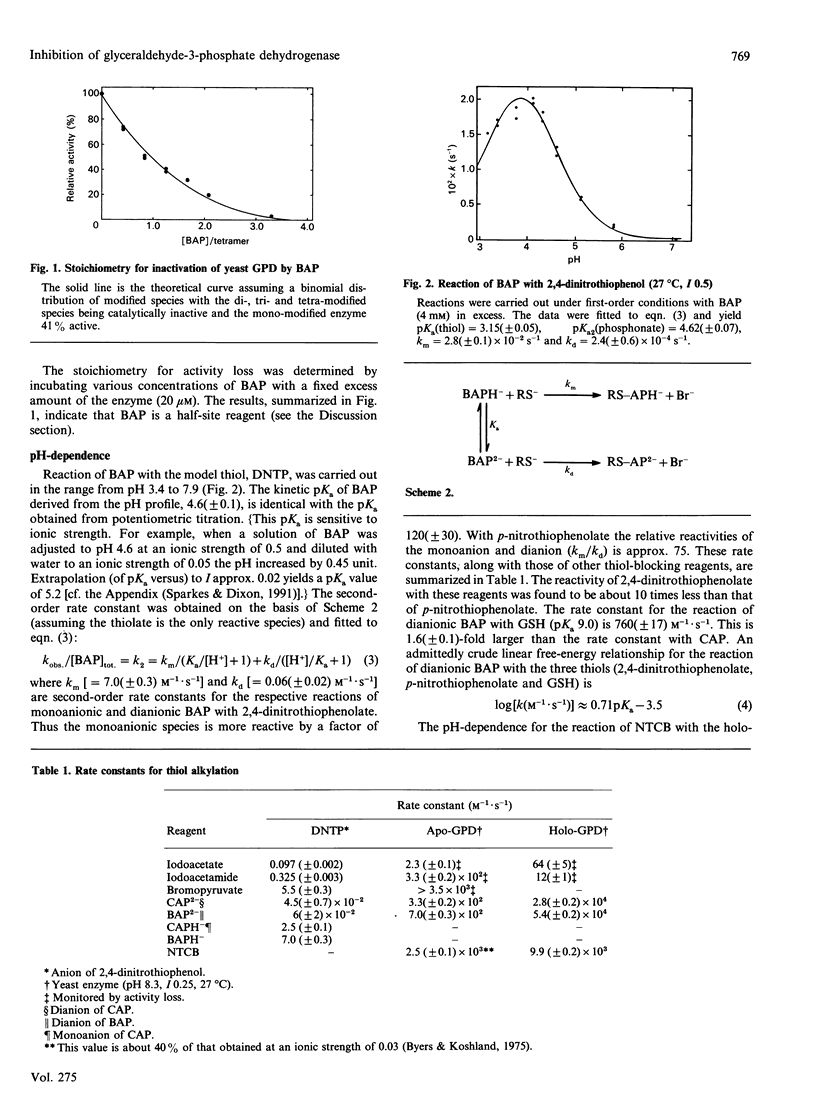
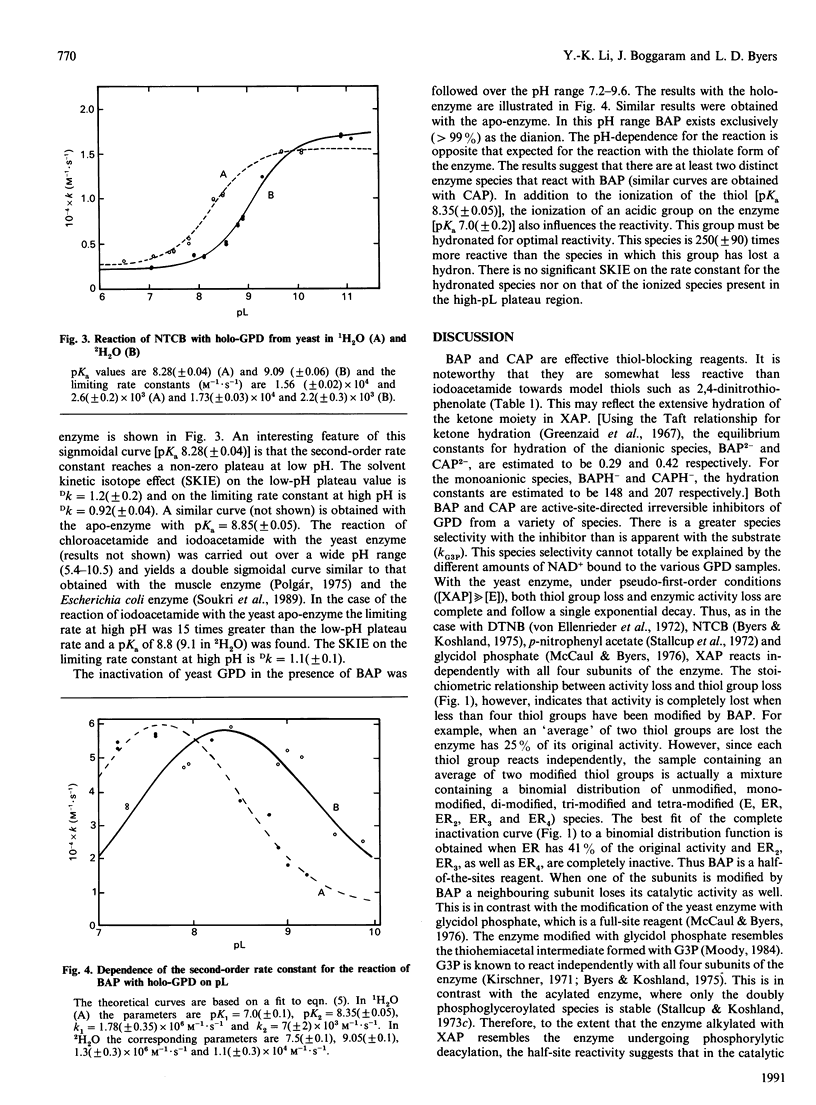
Two new alkylating reagents, chloro- and bromo-acetylphosphonate, were found to be very effective thiol-blocking reagents. The pH-dependence of the reaction of BAP with 2,4-dinitrothiophenol (25 degrees C, I 0.5) shows a tailing bell-shaped curve (with a plateau at high pH) characteristic of two ionizing groups: the thiol group (pKa 3.2) and the phosphonate group (pKa2 4.6). The rate constant for the reaction of the monoanionic inhibitor with dinitrothiophenolate (k2 = 7 M-1.s-1) is 120 times larger than that of the dianionic species. The haloacetylphosphonates were found to be irreversible inhibitors of glyceraldehyde-3-phosphate dehydrogenase from a variety of sources. They react with the active-site thiol group (Cys-149) and are half-site reagents with yeast glyceraldehyde-3-phosphate dehydrogenase. Thus, when two of the identical four subunits are modified the enzyme is catalytically inactive. The effects of pH (7-10), 2H2O and NAD+ on the reaction with the yeast enzyme were examined in detail. NAD+ enhances the alkylation rates. The second-order rate constant does not show a simple sigmoidal dependence on pH but rather a tailing bell-shaped curve (pKa 7.0 and 8.4) qualitatively similar to that obtained with dinitrothiophenol. There is no significant solvent isotope effect on the limiting rate constants and a normal isotope effect on the two pKa values. The results are consistent with the more reactive enzyme species containing a thiolate and an acidic group that may either donate a proton to the dianionic haloacetylphosphonate or orient the inhibitor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bednar R. A. Reactivity and pH dependence of thiol conjugation to N-ethylmaleimide: detection of a conformational change in chalcone isomerase. Biochemistry. 1990 Apr 17;29(15):3684–3690. doi: 10.1021/bi00467a014. [DOI] [PubMed] [Google Scholar]
- Buehner M., Ford G. C., Moras D., Olsen K. W., Rossmann M. G. Structure determination of crystalline lobster D-glyceraldehyde-3-phosphate dehydrogenase. J Mol Biol. 1974 Feb 5;82(4):563–585. doi: 10.1016/0022-2836(74)90249-6. [DOI] [PubMed] [Google Scholar]
- Byers L. D., Koshland D. E., Jr The specificity of induced conformational changes. The case of yeast glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1975 Aug 12;14(16):3661–3669. doi: 10.1021/bi00687a023. [DOI] [PubMed] [Google Scholar]
- Byers L. D., She H. S., Alayoff A. Interaction of phosphate analogues with glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1979 Jun 12;18(12):2471–2480. doi: 10.1021/bi00579a006. [DOI] [PubMed] [Google Scholar]
- Cardon J. W., Boyer P. D. Subunit interaction in catalysis. Some experimental and theoretical approaches with glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1982 Jul 10;257(13):7615–7622. [PubMed] [Google Scholar]
- Harrigan P. J., Trentham D. R. Kinetic studies on oxidized nicotinamide--adenine dinucleotide-facilitated reactions of D-glyceraldehyde 3-phosphate dehydrogenase. Biochem J. 1974 Nov;143(2):353–363. doi: 10.1042/bj1430353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchuger M. S., Leong P. M., Byers L. D. Interaction of the substrate phosphate substituent with glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1979 Oct 2;18(20):4373–4379. doi: 10.1021/bi00587a017. [DOI] [PubMed] [Google Scholar]
- Kirschner K. Co-operative binding of nicotinamide-adenine dinucleotide to yeast glyceraldehyde-3-phosphate dehydrogenase. II. Stopped-flow studies at pH 8-5 and 40 degrees C. J Mol Biol. 1971 May 28;58(1):51–68. doi: 10.1016/0022-2836(71)90231-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr The role of negative cooperativity and half-of-the-sites reactivity in enzyme regulation. Curr Top Cell Regul. 1976;10:1–40. doi: 10.1016/b978-0-12-152810-2.50008-5. [DOI] [PubMed] [Google Scholar]
- Little G., Brocklehurst K. Kinetics of the reversible reaction of papain with 5,5'-dithiobis-(2-nitrobenzoate) dianion: evidence for nucleophilic reactivity in the un-ionized thiol group of cysteine-25 and for general acid catalysis by histidine-159 of the reaction of the 5-mercapto-2-nitrobenzoate dianion with the papain-5-mercapto-2-nitrobenzoate mixed disulphide. Biochem J. 1972 Jun;128(2):475–477. doi: 10.1042/bj1280475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra O. P., Bernhard S. A. Spectrophotometric identification of an active site-specific acyl glyceraldehyde 3-phosphate dehydrogenase. The regulation of its kinetic and equilibrium properties by coenzyme. J Biol Chem. 1968 Mar 25;243(6):1243–1252. [PubMed] [Google Scholar]
- McCaul S., Byers L. D. The reaction of epoxides with yeast glyceraldehyde--3--phosphate dehydrogenase. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1028–1034. doi: 10.1016/s0006-291x(76)80235-5. [DOI] [PubMed] [Google Scholar]
- McComb R. B., Bond L. W., Burnett R. W., Keech R. C., Bowers G. N., Jr Determination of the molar absorptivity of NADH. Clin Chem. 1976 Feb;22(2):141–150. [PubMed] [Google Scholar]
- Munroe W. A., Lewis C. A., Jr, Dunlap R. B. Kinetics of sulfhydryl group modification of thymidylate synthetase: a proposal for activation of catalytic cysteinly residues. Biochem Biophys Res Commun. 1978 Jan 30;80(2):355–360. doi: 10.1016/0006-291x(78)90684-8. [DOI] [PubMed] [Google Scholar]
- Polgár L. Ion-pair formation as a source of enhanced reactivity of the essential thiol group of D-glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1975 Feb 3;51(1):63–71. doi: 10.1111/j.1432-1033.1975.tb03907.x. [DOI] [PubMed] [Google Scholar]
- SEGAL H. L., BOYER P. D. The role of sulfhydryl groups in the activity of D-glyceraldehyde 3-phosphate dehydrogenase. J Biol Chem. 1953 Sep;204(1):265–281. [PubMed] [Google Scholar]
- Soukri A., Mougin A., Corbier C., Wonacott A., Branlant C., Branlant G. Role of the histidine 176 residue in glyceraldehyde-3-phosphate dehydrogenase as probed by site-directed mutagenesis. Biochemistry. 1989 Mar 21;28(6):2586–2592. doi: 10.1021/bi00432a036. [DOI] [PubMed] [Google Scholar]
- Sparkes M. J., Dixon H. B. The preparation and properties of bromoacetylphosphonic acid. Biochem J. 1991 May 1;275(Pt 3):772–773. doi: 10.1042/bj2750772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup W. B., Koshland D. E., Jr Half-of-the sites reactivity and negative co-operativity: the case of yeast glyceraldehyde 3-phosphate dehydrogenase. J Mol Biol. 1973 Oct 15;80(1):41–62. doi: 10.1016/0022-2836(73)90232-5. [DOI] [PubMed] [Google Scholar]
- Stallcup W. B., Koshland D. E., Jr Half-of-the sites reactivity in the catalytic mechanism of yeast glyceraldehyde 3-phosphate dehydrogenase. J Mol Biol. 1973 Oct 15;80(1):77–91. doi: 10.1016/0022-2836(73)90234-9. [DOI] [PubMed] [Google Scholar]
- Stallcup W. B., Koshland D. E., Jr Reactive lysines of yeast glyceraldehyde 3-phosphate dehydrogenase. Attachment of a reporter group to a specific non-essential residue. J Mol Biol. 1973 Oct 15;80(1):63–75. doi: 10.1016/0022-2836(73)90233-7. [DOI] [PubMed] [Google Scholar]
- Stallcup W. B., Mockrin S. C., Koshland D. E., Jr A rapid purification procedure for glyceraldehyde 3-phosphate dehydrogenase from Bakers' yeast. J Biol Chem. 1972 Oct 10;247(19):6277–6279. [PubMed] [Google Scholar]
- Trentham D. R. Rate-determining processes and the number of simultaneously active sties of D-glyceraldehyde 3-phosphate dehydrogenase. Biochem J. 1971 Mar;122(1):71–77. doi: 10.1042/bj1220071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VELICK S. F., HAYES J. E., Jr Phosphate binding and the glyceraldehyde-3-phosphate dehydrogenase reaction. J Biol Chem. 1953 Aug;203(2):545–562. [PubMed] [Google Scholar]
- Wrba A., Schweiger A., Schultes V., Jaenicke R., Závodszky P. Extremely thermostable D-glyceraldehyde-3-phosphate dehydrogenase from the eubacterium Thermotoga maritima. Biochemistry. 1990 Aug 21;29(33):7584–7592. doi: 10.1021/bi00485a007. [DOI] [PubMed] [Google Scholar]
- von Ellenrieder G., Kirschner K., Schuster I. The binding of oxidized and reduced nicotinamide adenine-dinucleotide to yeast glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1972 Mar 27;26(2):220–236. doi: 10.1111/j.1432-1033.1972.tb01760.x. [DOI] [PubMed] [Google Scholar]


