Abstract
Major histocompatibility complex class II-mediated antigen presentation after intranasal infection with murine gammaherpesvirus 68 differs in mediastinal lymph nodes and spleen. Evidence that virus-specific CD4+ T cells were being stimulated was found as late as 6 to 8 months after infection, and cells specific for the viral gp15067–83 and ORF11168–180 peptides were maintained as a fairly stable proportion of the total response.
The murine gammaherpesvirus 68 (γHV68) provides a unique model for the experimental dissection of immunity to a DNA virus that persists in lymphoid tissue (21, 25, 38). While most of the studies have focused on the CD8+ T-cell response to human γHV (6, 22), the characteristics of CD4+ T-cell-mediated immunity have generally received less attention. The results of recent experiments have shown, however, that gamma interferon (IFN-γ) production by CD4+ T cells plays an important, protective role in γHV68 infection (8). Vaccination with gp15067–83, a γHV68 peptide presented by the H2I-Ab major histocompatibility complex (MHC) class II glycoprotein, resulted in some reduction of virus replication in the respiratory tract of mice challenged intranasally (i.n.), though there was no effect on the extent of latency in the lymphoid tissue (20). Mice that are CD4+ T cell deficient as a consequence of disruption of the MHC class II glycoprotein (I-Ab−/−) eventually succumb within 120 days of the initial exposure to γHV68 (7). Furthermore, experiments with CD4-depleted and CD40L−/− mice have established that CD4+ T help is essential for both the expansion of the CD8+ Vβ4+ set that characterizes this infection and the development of class-switched, γHV68-specific, and nonspecific immunoglobulin responses (4, 15, 24, 28).
In addition, though massively expanded CD8+ T-cell populations clearly dominate the response to γHV68 for the first 30 days after γHV68 challenge (26, 34), the numbers of CD4+ T cells in both blood and lymphoid tissue are also greatly increased (12, 39). This reflects enhanced proliferation of the “activated” CD4+ CD44hi (high level of CD44) set, which continues for at least 3 weeks after the initial exposure to virus (18). The present experiments look more closely at the nature and characteristics of antigen expression in vivo for γHV68-specific CD4+ T cells and quantify the CD4+ T-cell response to two H2I-Ab-restricted peptides in the long term.
A new γHV68 peptide presented by H21-Ab and the kinetics of antigen expression.
The IFN-γ enzyme-linked immunospot (ELISpot) assay (5, 9) was used to screen peptide response profiles for CD4+ T cells enriched from the spleens of C57BL/6J (B6) mice that had been infected i.n. with 600 PFU of γHV68 21 or 100 days previously. The peptides were either selected from the entire genome of γHV68 as 13-mers conforming to the I-Ab motif (XXNXXXXXPXX), or were complete sets (15-mers overlapping by 10 amino acids) from the M3, M9, ORF11, ORF72, ORF73, and ORF74 proteins (23, 37). The positive control was the previously defined H2I-Ab gp15067–83 epitope (20). Pooled peptides that gave a positive response were then tested singly using peptide-pulsed, T-cell-depleted spleen cells from uninfected mice and H2I-Ab-transfected L cells (H-2k) as antigen-presenting cells (APCs). This extensive analysis identified only one new peptide at 20 μM, ORF11168–180 (TFKNFNTATPSLE). The function of the γHV68 ORF11 is unknown, although the gene is relatively conserved in the γHVs, sharing approximately 20% identity with homologues in Epstein-Barr virus–RajiLF2, human herpesvirus 8, and herpes simplex virus (37).
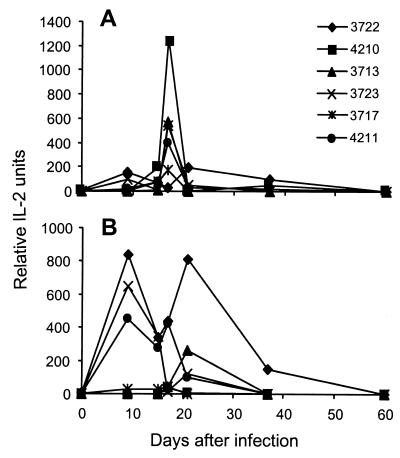
A panel of five CD4+ T-cell hybridomas was derived (10, 19, 20) from the mediastinal lymph nodes (MLNs) of γHV68-infected mice. All these hybridomas could be stimulated with the γHV68-infected, H2I-Ab-transfected L-cell line, but none responded to ORF11168–180 or gp15067–83. We then used all five, together with the gp15067–83-specific T-cell hybridoma (4211) previously described (20), to probe the kinetics of antigen presentation in lymphoid tissue after i.n. infection with γHV68 (19, 36). Stimulatory activity mediated by APCs recovered directly from the infected mice was maximal at about day 17 after infection for the spleen and subsequently declined rapidly (Fig. 1A). In contrast, viral antigen presentation in the MLN was detected earlier and sustained from days 9 to 20 (Fig. 1B). None of these hybridomas was stimulated by potential APC populations isolated subsequent to day 60 after infection (data not shown).
FIG. 1.
Kinetics of H2I-Ab-restricted antigen expression in the spleen (A) and MLN (B) during γHV68 infection. Anesthetized mice were infected i.n. with 600 PFU of γHV68 and then sampled at intervals to isolate APCs from the MLN and spleen for assay with hybridoma T-cell lines (7, 19). The 4211 hybridoma is specific for the gp15067–83 peptide (21), while the other hybridomas are known to respond only to γHV68-infected L cells transfected with H2I-Ab. The experimental protocols have been described in detail previously (19, 31, 40). These data are representative of two independent experiments, with each time point showing data for cells pooled from three mice. IL-2, interleukin 2.
These results (Fig. 1) presumably reflect the staged expression of γHV68 proteins and are generally comparable to those found in an earlier study of MHC class I-restricted antigen presentation for the γHV68-specific CD8+ subset (19, 36). The greater prominence of APCs in the MLN at the day 9 time point could reflect the fact that the regional lymph nodes are the primary homing site for antigen-presenting dendritic cells entering afferent lymph from the lung, the principal site of replicative γHV68 infection (7, 21).
Quantitation of the γHV68-specific CD4+ T-cell response.
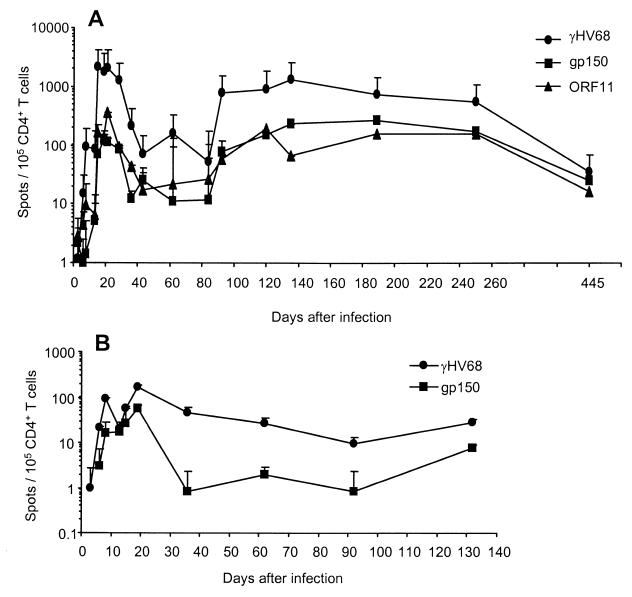
We repeated a more detailed ELISpot analysis of the acute and long-term CD4+ T-cell response that had been done previously with γHV68-infected APCs (9), adding peptide-pulsed stimulators to the equation. The frequencies of CD4+ T cells specific for γHV68 (1/48), gp15067–83 (1/870), and ORF11168–180 (1/279) peaked in the spleen around day 21 after infection. These values fell 5- to 10-fold between days 40 and 80, then increased again, and remained fairly stable over an 8-month period (average frequency of 1/117 from days 90 to 250). The continued presence of the gp15067–83-specific population was also demonstrated in the MLN through day 130 after infection (Fig. 2B), but the analysis was less detailed and was not continued because of the small numbers of lymphocytes obtained, especially at the later time points.
FIG. 2.
Kinetic analysis of the antigen-specific response in enriched CD4+ T-cell populations recovered from the spleen (A) and MLN (B) of B6 mice infected i.n. with 600 PFU of γHV68. The IFN-γ ELISpot assay utilized T-cell-depleted APCs that had been infected with γHV68 (circles) or pulsed with 20 μM (each) gp15067–83 (squares) or ORF11168–180 (triangles) peptide. The results are expressed as the number of IFN-γ-producing cells per 105 CD4+ T cells, with each time point showing the mean ± standard deviation values for three to six mice from two to three independent experiments. The assay system used here has been described in detail previously (9).
The combined responses to the gp15067–83 and ORF11168–180 peptides generally accounted for 10 to 20% of the total γHV68-specific CD4+ T cells in the spleen between days 20 and 250 after infection (Fig. 2A). By day 445, however, the overall frequency of γHV68-specific CD4+ T cells had fallen to 1/2,848, while those reactive to the two peptides seemed to comprise the majority of the virus-specific set.
Cycling of virus-specific CD4+ T cells.
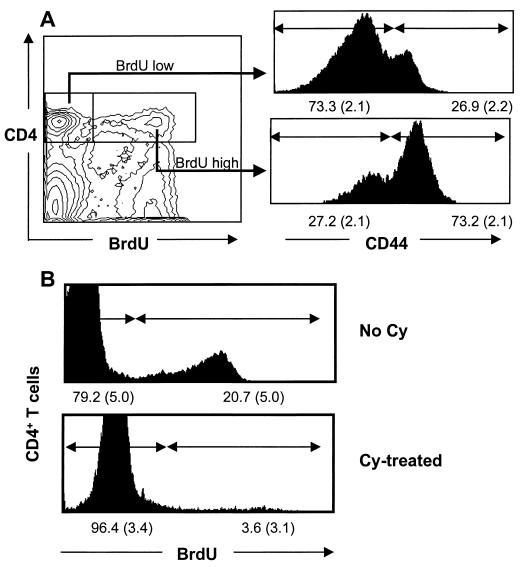
The numbers of γHV68-specific CD4+ T cells were maintained at a relatively high level in the 6 to 8 months after the initial virus challenge (Fig. 2), though we could no longer detect the presence of antigen in freshly isolated APCs with the hybridoma assay (Fig. 1 and data not shown). We thus asked whether we could find other evidence that these CD4+ T cells were indeed being continuously exposed to γHV68 peptides. Not surprisingly, a bromodeoxyuridine (BrdU)-feeding experiment (32, 33) showed that the majority of the CD4+ BrdUhi set that could be detected in the spleen at the 6 month time point were also CD44hi (Fig. 3A). This tells us that most of the T cells that incorporate BrdU in their DNA over a 6-day feeding period have an activated or memory phenotype (30) but does not in any sense establish that all (or any) of these lymphocytes are γHV68 specific.
FIG. 3.
CD44 staining profile for cycling CD4+ T cells (A) and elimination of this CD4+ BrdUhi population by Cy treatment (B). The numbers on the x axis in panel B show the means (standard deviations shown in parentheses) from three individual mice. The data shown are representative of two repeat experiments using B6 mice infected i.n. 6 to 8 months previously with 600 PFU of γHV68. The mice were fed water containing 0.8 mg of BrdU (Sigma) per ml for 6 to 8 days prior to sampling (3, 18, 33). Some mice were injected intraperitoneally with 50 mg of Cy per kg of body weight given every second day for 8 days.
The availability of tetramers that allow the direct staining of the antigen-specific population has greatly facilitated such analyses for virus-specific CD8+ T cells (2, 26, 27), but we lack such reagents for the γHV68-specific CD4+ set. We thus returned to an old protocol (1, 35), giving repeated, low doses of the alkylating agent cyclophosphamide (Cy) to determine whether exposure to this cell cycle-specific, cytotoxic drug would diminish the prevalence of the γHV68-specific CD4+ population. The net result was almost complete elimination of the CD4+ BrdUhi set from the γHV68-infected mice (Fig. 3B). Due to the low dose and short period of Cy treatment, we consider that collateral effects on γHV68-infected cells, although possible, are unlikely to affect frequencies of virus-specific CD4+ T cells. Frequency analysis with the ELISpot assay established that Cy treatment diminished the γHV68- and gp15067–83-specific responses by a factor of approximately 10-fold (Table 1). The effect was less obvious for the smaller ORF11168–180-specific response and was not seen at all (35) for influenza virus-specific CD4+ T cells from control mice that had been treated with Cy 2 months after being primed with the HKx31 influenza A virus (Table 1).
TABLE 1.
Consequences of Cy treatment for mice infected previously with γHV68 or the HKx31 influenza A virus
| In vivo priminga | In vitro stimulationb | Reciprocal of CD4+-T-cell frequencyc
|
|
|---|---|---|---|
| Control (no Cy) | Cy treatedd | ||
| γHV68 | γHV68 | 174 (55–333) | 3,286 (2,397–4,267) |
| γHV68 | gp15067–83 | 4,157 (2,344–5,691) | 46,979 (15,361–100,000) |
| γHV68 | ORF11168–180 | 35,520 (13,280–80,000) | 84,615 (76,923–100,000) |
| HKx31 | HKx31 | 4,834 (1,524–19,950) | 8,000 (3,221–7,918) |
B6 mice were infected i.n. with 600 PFU of γHV68 or 300 50% egg infective doses of the HKx31 influenza A virus and then sampled after an additional 6 to 8 months (γHV68) or 2 months (HKx31).
Enriched CD4+ T cells were stimulated with virus-infected or peptide-pulsed APCs and then analyzed by the IFN-γ ELISpot assay as described in the legend to Fig. 2.
The results are expressed as mean values (ranges shown in parentheses) from two separate experiments for mice primed with γHV68 and HKx31, respectively. Each experiment utilized cells pooled from three mice, which were analyzed in triplicate using serial twofold dilutions.
The mice were given 50 mg of Cy per kg intraperitoneally every second day for 8 days prior to sampling. Though the experimental protocol for the γHV68-infected mice was the same as that used for the BrdU staining experiment illustrated in Fig. 3, none of the mice used in these frequency studies had been given BrdU.
CD4+ T-cell “memory” to readily eliminated and persistent viruses.
The influenza A viruses are thought to be completely eliminated during the acute phase of the response (14, 17), while γHV68 can be recovered from the spleen in the long term by the cocultivation of viable lymphocytes and macrophages on susceptible monolayers (29, 39). However, it is generally not possible to isolate infectious virus from supernatants of spleen homogenates, probably because plaque assays are not sensitive enough to detect low frequencies of infectious virus (7). Even so, the continued cycling of CD4+ T cells specific for the gp15067–83 peptide in mice assayed 6 to 8 months after the initial exposure to γHV68 (Table 1) indicates that such reactivation to lytic phase must indeed be occurring. These results are comparable to γHV68-specific CD8+ T-cell proliferation described during the persistent phase of γHV infection (3).
These experiments thus support more-detailed studies of γHV68-specific and influenza virus-specific CD8+ T-cell responses, which indicate that the characteristics of T-cell memory to readily eliminated and persistent viruses differ (3, 16). This is hardly surprising but needs to be taken into account as we consider the potential life spans of antigen-reactive lymphocyte populations, especially in humans where clonal deletion as a consequence of telomere shortening constitutes a potential problem for virus control in the very long term (13). It is also debatable whether we should indeed refer to those lymphocytes that are continuously or sporadically exposed to restimulation from an endogenous antigen source as “memory” T cells (11).
Acknowledgments
We thank Kris Branum and Sherri Surman for technical assistance and Vicki Henderson for help with the manuscript.
These studies were supported in part by U.S. Public Health Service grants AI29579, AI38359, and CA21765 and by the American Lebanese Syrian Associated Charities (ALSAC).
REFERENCES
- 1.Allan J E, Doherty P C. Consequences of cyclophosphamide treatment in murine lymphocytic choriomeningitis: evidence for cytotoxic T cell replication in vivo. Scand J Immunol. 1985;22:367–374. doi: 10.1111/j.1365-3083.1985.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 2.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 3.Belz G T, Doherty P C. Virus-specific and bystander CD8+ T-cell proliferation in the acute and persistent phases of a gammaherpesvirus infection. J Virol. 2001;75:4435–4438. doi: 10.1128/JVI.75.9.4435-4438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks J W, Hamilton-Easton A M, Christensen J P, Cardin R D, Hardy C L, Doherty P C. Requirement for CD40 ligand, CD4+ T cells, and B cells in an infectious mononucleosis-like syndrome. J Virol. 1999;73:9650–9654. doi: 10.1128/jvi.73.11.9650-9654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butz E A, Bevan M J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callan M F, Tan L, Annels N, Ogg G S, Wilson J D, O'Callaghan C A, Steven N, McMichael A J, Rickinson A B. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardin R D, Brooks J W, Sarawar S R, Doherty P C. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen J P, Cardin R D, Branum K C, Doherty P C. CD4+ T cell-mediated control of a gamma-herpesvirus in B cell-deficient mice is mediated by IFN-gamma. Proc Natl Acad Sci USA. 1999;96:5135–5140. doi: 10.1073/pnas.96.9.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen J P, Doherty P C. Quantitative analysis of the acute and long-term CD4+ T-cell response to a persistent gammaherpesvirus. J Virol. 1999;73:4279–4283. doi: 10.1128/jvi.73.5.4279-4283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coppola M A, Flaño E, Nguyen P, Hardy C L, Cardin R D, Shastri N, Woodland D L, Blackman M A. Apparent MHC-independent stimulation of CD8+ T cells in vivo during latent murine gammaherpesvirus infection. J Immunol. 1999;163:1481–1489. [PubMed] [Google Scholar]
- 11.Doherty P C. The terminology problem for T cells: a discussion paper. Philos Trans R Soc Lond B. 2000;355:361–362. doi: 10.1098/rstb.2000.0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doherty P C, Hamilton-Easton A M, Topham D J, Riberdy J, Brooks J W, Cardin R D. Consequences of viral infections for lymphocyte compartmentalization and homeostasis. Semin Immunol. 1997;9:365–373. doi: 10.1006/smim.1997.0094. [DOI] [PubMed] [Google Scholar]
- 13.Effros R B, Pawelec G. Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion? Immunol Today. 1997;18:450–454. doi: 10.1016/s0167-5699(97)01079-7. [DOI] [PubMed] [Google Scholar]
- 14.Eichelberger M C, Wang M, Allan W, Webster R G, Doherty P C. Influenza virus RNA in the lung and lymphoid tissue of immunologically intact and CD4-depleted mice. J Gen Virol. 1991;72:1695–1698. doi: 10.1099/0022-1317-72-7-1695. [DOI] [PubMed] [Google Scholar]
- 15.Flaño E, Woodland D L, Blackman M A. Requirement for CD4+ T cells in Vβ4+CD8+ T cell activation associated with latent murine gammaherpesvirus infection. J Immunol. 1999;163:3403–3408. [PubMed] [Google Scholar]
- 16.Flynn K J, Riberdy J M, Christensen J P, Altman J D, Doherty P C. In vivo proliferation of naive and memory influenza-specific CD8+ T cells. Proc Natl Acad Sci USA. 1999;96:8597–8602. doi: 10.1073/pnas.96.15.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton-Easton A, Eichelberger M. Virus-specific antigen presentation by different subsets of cells from lung and mediastinal lymph node tissues of influenza virus-infected mice. J Virol. 1995;69:6359–6366. doi: 10.1128/jvi.69.10.6359-6366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton-Easton A M, Christensen J P, Doherty P C. Turnover of T cells in murine gammaherpesvirus 68-infected mice. J Virol. 1999;73:7866–7869. doi: 10.1128/jvi.73.9.7866-7869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Flaño E, Usherwood E J, Surman S, Blackman M A, Woodland D L. Lytic cycle T cell epitopes are expressed in two distinct phases during MHV-68 infection. J Immunol. 1999;163:868–874. [PubMed] [Google Scholar]
- 20.Liu L, Usherwood E J, Blackman M A, Woodland D L. T-cell vaccination alters the course of murine herpesvirus 68 infection and the establishment of viral latency in mice. J Virol. 1999;73:9849–9857. doi: 10.1128/jvi.73.12.9849-9857.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nash A A, Sunil-Chandra N P. Interactions of the murine gammaherpesvirus with the immune system. Curr Opin Immunol. 1994;6:560–563. doi: 10.1016/0952-7915(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 22.Rickinson A B, Moss D J. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu Rev Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- 23.Rudensky A Y, Preston-Hurlburt P, Hong S C, Barlow A, Janeway C A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991;353:622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- 24.Sangster M Y, Topham D J, D'Costa S, Cardin R D, Marion T N, Myers L K, Doherty P C. Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. J Immunol. 2000;164:1820–1828. doi: 10.4049/jimmunol.164.4.1820. [DOI] [PubMed] [Google Scholar]
- 25.Simas J P, Efstathiou S. Murine gammaherpesvirus 68: a model for the study of gammaherpesvirus pathogenesis. Trends Microbiol. 1998;6:276–282. doi: 10.1016/s0966-842x(98)01306-7. [DOI] [PubMed] [Google Scholar]
- 26.Stevenson P G, Belz G T, Altman J D, Doherty P C. Changing patterns of dominance in the CD8+ T cell response during acute and persistent murine gamma-herpesvirus infection. Eur J Immunol. 1999;29:1059–1067. doi: 10.1002/(SICI)1521-4141(199904)29:04<1059::AID-IMMU1059>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson P G, Belz G T, Castrucci M R, Altman J D, Doherty P C. A gamma-herpesvirus sneaks through a CD8+ T cell response primed to a lytic-phase epitope. Proc Natl Acad Sci USA. 1999;96:9281–9286. doi: 10.1073/pnas.96.16.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevenson P G, Cardin R D, Christensen J P, Doherty P C. Immunological control of a murine gammaherpesvirus independent of CD8+ T cells. J Gen Virol. 1999;80:477–483. doi: 10.1099/0022-1317-80-2-477. [DOI] [PubMed] [Google Scholar]
- 29.Sunil-Chandra N P, Efstathiou S, Nash A A. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J Gen Virol. 1992;73:3275–3279. doi: 10.1099/0022-1317-73-12-3275. [DOI] [PubMed] [Google Scholar]
- 30.Tabi Z, Lynch F, Ceredig R, Allan J E, Doherty P C. Virus-specific memory T cells are Pgp-1+ and can be selectively activated with phorbol ester and calcium ionophore. Cell Immunol. 1988;113:268–277. doi: 10.1016/0008-8749(88)90026-3. [DOI] [PubMed] [Google Scholar]
- 31.Topham D J, Doherty P C. Longitudinal analysis of the acute Sendai virus-specific CD4+ T cell response and memory. J Immunol. 1998;161:4530–4535. [PubMed] [Google Scholar]
- 32.Tough D F, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tough D F, Sprent J. Lifespan of γδT cells. J Exp Med. 1998;187:357–365. doi: 10.1084/jem.187.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tripp R A, Hamilton-Easton A M, Cardin R D, Nguyen P, Behm F G, Woodland D L, Doherty P C, Blackman M A. Pathogenesis of an infectious mononucleosis-like disease induced by a murine gamma-herpesvirus: role for a viral superantigen? J Exp Med. 1997;185:1641–1650. doi: 10.1084/jem.185.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tripp R A, Hou S, McMickle A, Houston J, Doherty P C. Recruitment and proliferation of CD8+ T cells in respiratory virus infections. J Immunol. 1995;154:6013–6021. [PubMed] [Google Scholar]
- 36.Usherwood E J, Roy D J, Ward K, Surman S L, Dutia B M, Blackman M A, Stewart J P, Woodland D L. Control of γ herpesvirus latency by latent antigen-specific CD8+ T cells. J Exp Med. 2000;192:943–952. doi: 10.1084/jem.192.7.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Virgin H W, IV, Latreille P, Wamsley P, Hallsworth K, Weck K E, Dal Canto A J, Speck S H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Virgin H W, Speck S H. Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection. Curr Opin Immunol. 1999;11:371–379. doi: 10.1016/s0952-7915(99)80063-6. [DOI] [PubMed] [Google Scholar]
- 39.Weck K E, Kim S S, Virgin H W I, Speck S H. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J Virol. 1999;73:3273–3283. doi: 10.1128/jvi.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodland D, Happ M P, Bill J, Palmer E. Requirement for cotolerogenic gene products in the clonal deletion of I-E reactive T cells. Science. 1990;247:964–967. doi: 10.1126/science.1968289. [DOI] [PubMed] [Google Scholar]