Abstract
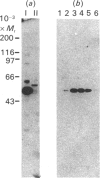
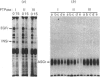
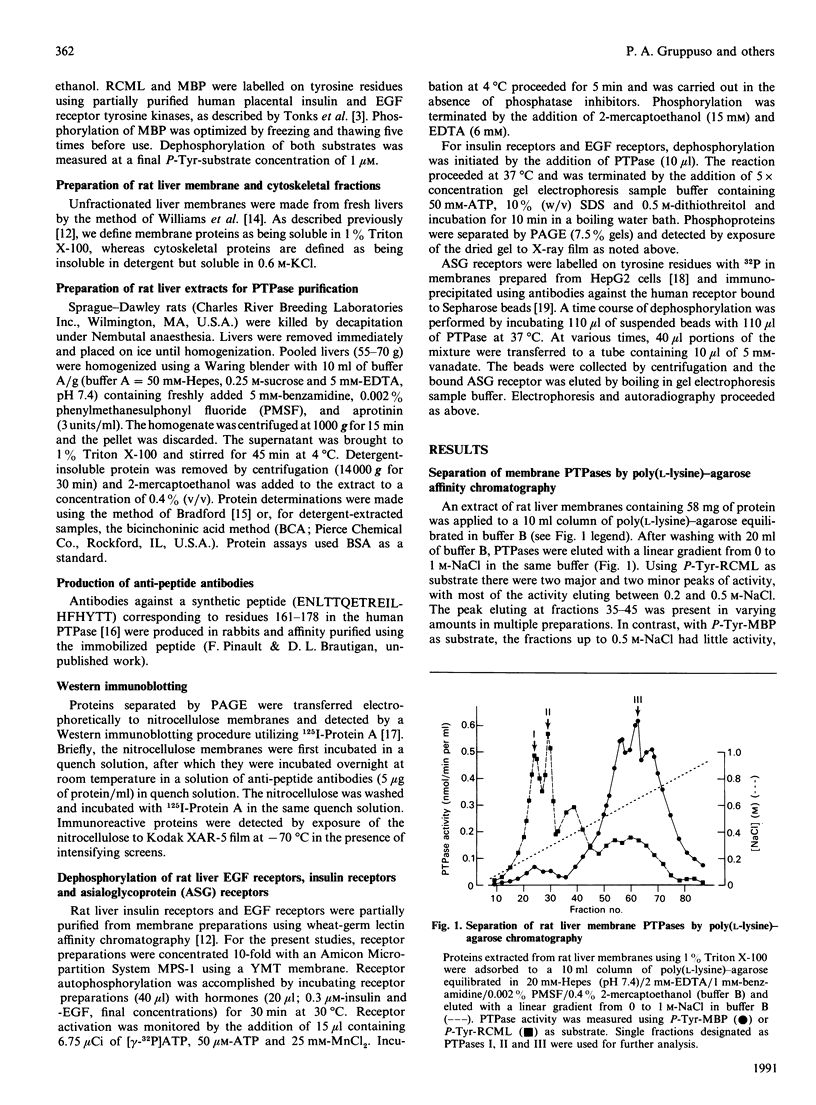
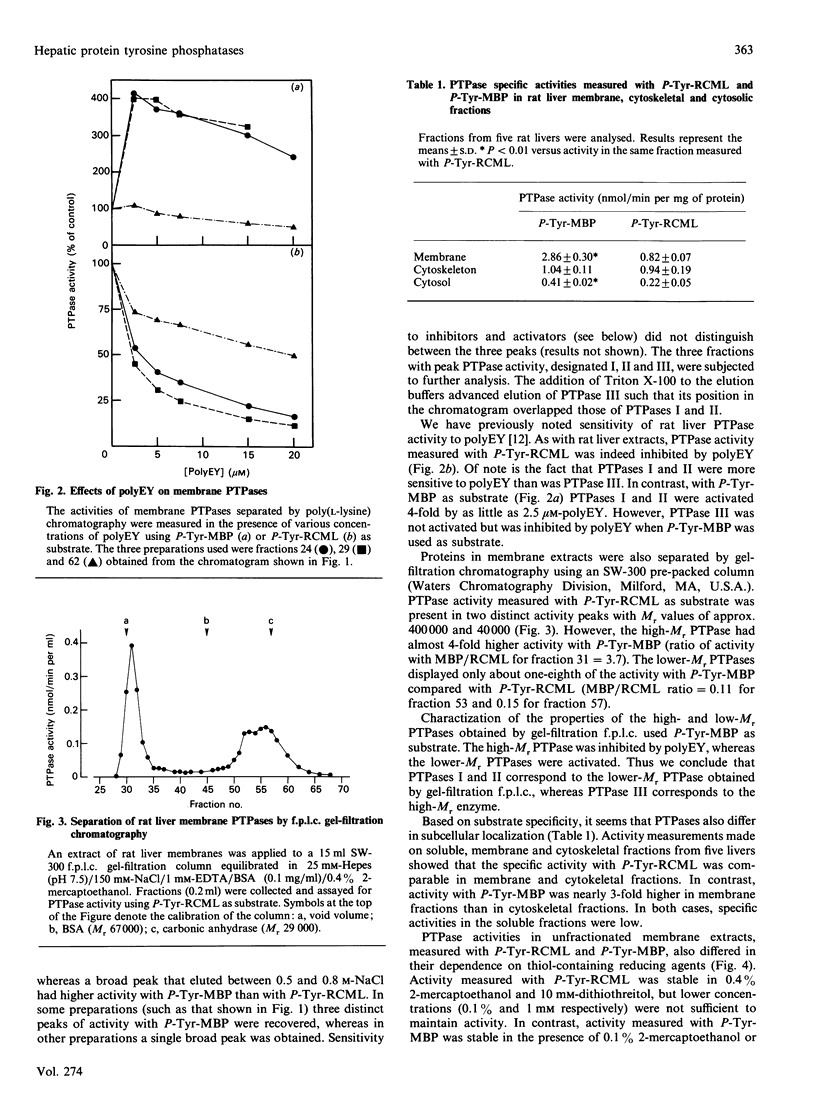
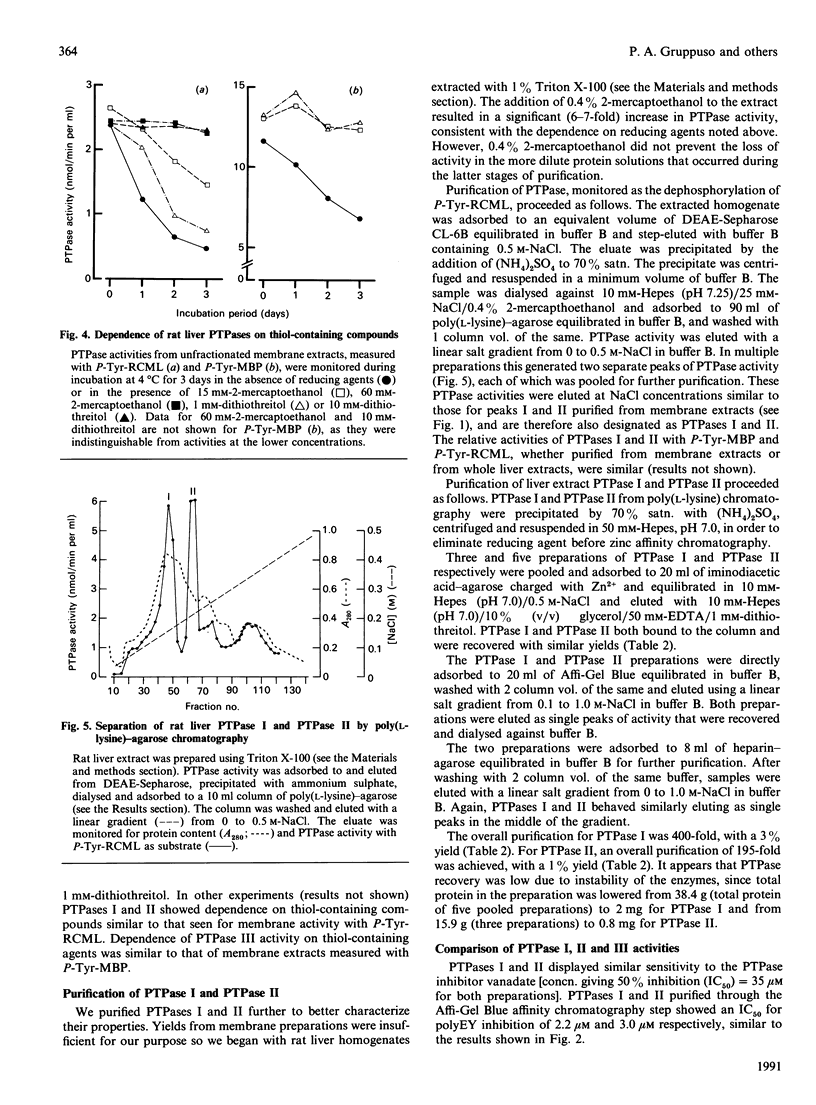
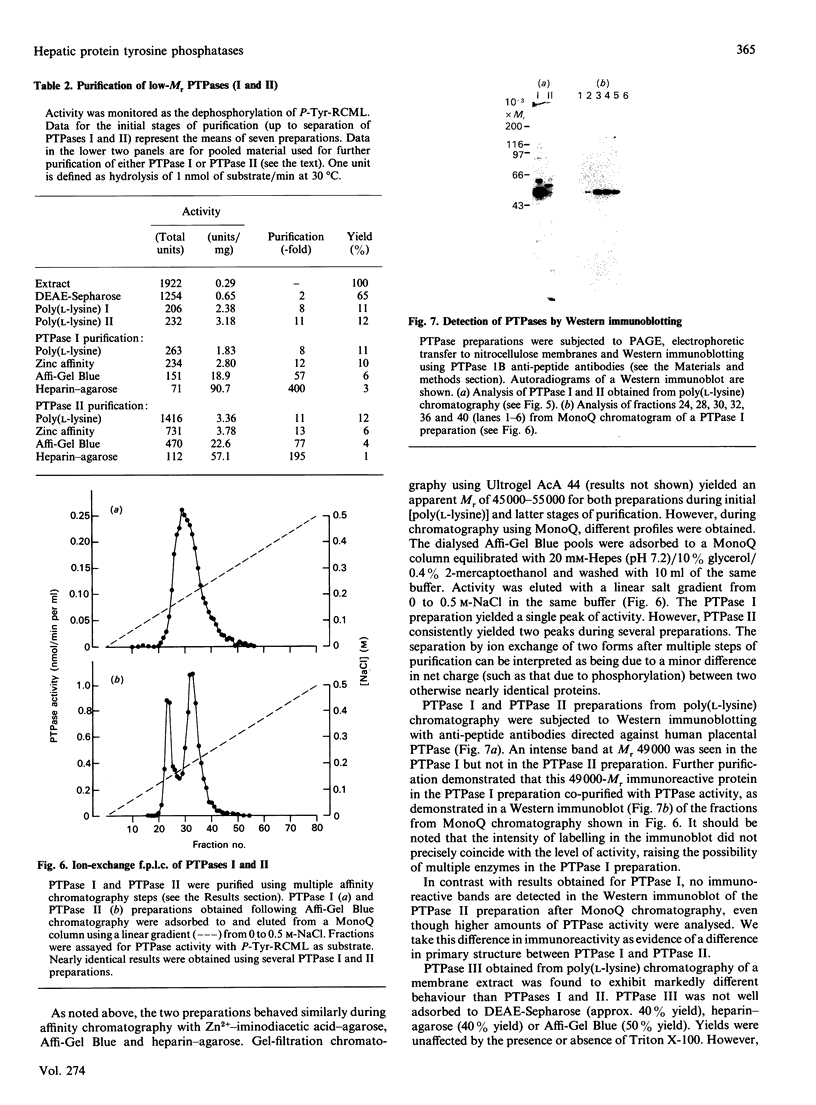
Regulation of cell growth and metabolism by protein tyrosine phosphorylation involves dephosphorylation via the action of protein tyrosine phosphatases (PTPases). We have characterized the membrane PTPases in rat liver, monitoring their activity by measuring the dephosphorylation of P-Tyr-reduced, carboxyamidomethylated and maleylated lysozyme (P-Tyr-RCML) and P-Tyr-myelin basic protein (P-Tyr-MBP). Separation of membrane PTPases by poly (L-lysine) chromatography yielded three peaks of PTPase, termed I, II and III. PTPases I and II were most active with P-Tyr-RCML, whereas PTPase III showed greater activity with P-Tyr-MBP than with P-Tyr-RCML (ratio of activities 4:1). Separation of membrane proteins by gel-filtration chromatography yielded two peaks of activity. Based on substrate specificity, sensitivity to inhibitors and requirement for thiol-containing compounds, the activity peak with an Mr of approximately 400,000 corresponded to PTPase III, whereas that with an Mr of approx. 40,000 contained PTPases I and II. All three PTPases dephosphorylated epidermal growth factor receptors and insulin receptors, but only PTPases I and II were active with P-Tyr-asialoglycoprotein receptors. Although none of the above characteristics distinguished between PTPases I and II, only PTPase I reacted in a Western immunoblotting procedure with anti-peptide antibodies directed towards human placental PTPase. We conclude that the membrane fraction from rat liver contains at least three distinct PTPases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Gruppuso P. A., Mumby M. Protein phosphatase type-1 and type-2 catalytic subunits both bind inhibitor-2 and monoclonal immunoglobulins. J Biol Chem. 1986 Nov 15;261(32):14924–14928. [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K., Kumar S., Diltz C. D., Harrylock M., Cool D. E., Krebs E. G., Fischer E. H., Walsh K. A. Human placenta protein-tyrosine-phosphatase: amino acid sequence and relationship to a family of receptor-like proteins. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5252–5256. doi: 10.1073/pnas.86.14.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K., Walsh K. A., Fischer E. H. The leukocyte common antigen (CD45): a putative receptor-linked protein tyrosine phosphatase. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7182–7186. doi: 10.1073/pnas.85.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff J., Schievella A. R., Jost C. A., Erikson R. L., Neel B. G. Cloning of a cDNA for a major human protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2735–2739. doi: 10.1073/pnas.87.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool D. E., Tonks N. K., Charbonneau H., Walsh K. A., Fischer E. H., Krebs E. G. cDNA isolated from a human T-cell library encodes a member of the protein-tyrosine-phosphatase family. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5257–5261. doi: 10.1073/pnas.86.14.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon R. J., Schwartz A. L. Regulation by phorbol esters of asialoglycoprotein and transferrin receptor distribution and ligand affinity in a hepatoma cell line. J Biol Chem. 1986 Nov 15;261(32):15081–15089. [PubMed] [Google Scholar]
- Fallon R. J. Tyrosine phosphorylation of the asialoglycoprotein receptor. J Biol Chem. 1990 Feb 25;265(6):3401–3406. [PubMed] [Google Scholar]
- Gruppuso P. A., Boylan J. M., Posner B. I., Faure R., Brautigan D. L. Hepatic protein phosphotyrosine phosphatase. Dephosphorylation of insulin and epidermal growth factor receptors in normal and alloxan diabetic rats. J Clin Invest. 1990 Jun;85(6):1754–1760. doi: 10.1172/JCI114632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Haun R. S., Watson S. J., Geahlen R. L., Dixon J. E. Cloning and expression of a protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1501–1505. doi: 10.1073/pnas.87.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Jove R., Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- Shriner C. L., Brautigan D. L. Cytosolic protein phosphotyrosine phosphatases from rabbit kidney. Purification of two distinct enzymes that bind to Zn2+-iminodiacetate agarose. J Biol Chem. 1984 Sep 25;259(18):11383–11390. [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Hall L. R., Schlossman S. F., Saito H. A new member of the immunoglobulin superfamily that has a cytoplasmic region homologous to the leukocyte common antigen. J Exp Med. 1988 Nov 1;168(5):1523–1530. doi: 10.1084/jem.168.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. L. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Charbonneau H., Diltz C. D., Fischer E. H., Walsh K. A. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry. 1988 Nov 29;27(24):8695–8701. doi: 10.1021/bi00424a001. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. CD45, an integral membrane protein tyrosine phosphatase. Characterization of enzyme activity. J Biol Chem. 1990 Jun 25;265(18):10674–10680. [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Characterization of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6731–6737. [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Purification of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6722–6730. [PubMed] [Google Scholar]
- Williams L. T., Tremble P. M., Lavin M. F., Sunday M. E. Platelet-derived growth factor receptors form a high affinity state in membrane preparations. Kinetics and affinity cross-linking studies. J Biol Chem. 1984 Apr 25;259(8):5287–5294. [PubMed] [Google Scholar]