Abstract
The enzyme ornithine decarboxylase (ODC, EC 4.1.1.17) is believed to play an essential role in the growth and differentiation of cells by regulating the biosynthesis of polyamines. The 5' untranslated region (5' UTR) of the ODC mRNA of different species is rather unusual in length and GC content, and may therefore be involved in translational control of ODC protein synthesis. We cloned the rat ODC cDNA downstream of the phage T7 promoter in order to perform transcription/translation studies in vitro. Our results show that the intact 5' UTR of rat ODC mRNA, which is 303 nt in length, is a potent inhibitor of translation. Efficient synthesis in vitro of ODC protein is obtained when either 172 nt from the 5'-end or 236 nt from the 3'-end of the 5' UTR are removed. A truncated 5' UTR with a calculated free energy of less than -272 kJ (-65 kcal/mol) is unable to support the synthesis in vitro of ODC protein. The short open reading frame (ORF) present in the 5' UTR of rat ODC mRNA does not contribute to the observed inhibitory effect on translation efficiency in vitro. At low polyamine concentration the efficiency of translation in vitro of intact ODC mRNA is not relatively increased compared with that of an ODC mRNA having a truncated 5' UTR or with that of control globin mRNA. From this we conclude that the well-documented negative feedback control of intracellular polyamines on ODC expression is not regulated by effects of polyamines on the secondary structure of the 5' UTR of ODC mRNA.
Full text
PDF

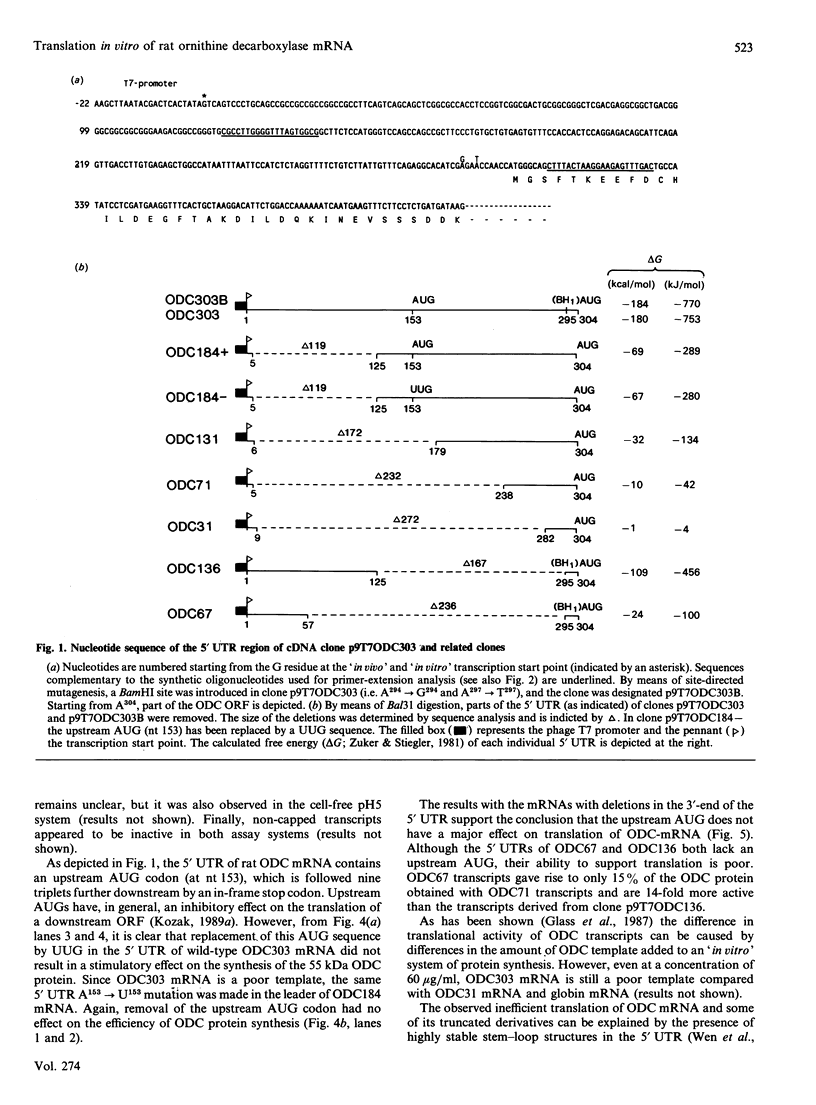



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger F. G., Loose D., Meisner H., Watson G. Androgen induction of messenger RNA concentrations in mouse kidney is posttranscriptional. Biochemistry. 1986 Mar 11;25(5):1170–1175. doi: 10.1021/bi00353a034. [DOI] [PubMed] [Google Scholar]
- Berger F. G., Szymanski P., Read E., Watson G. Androgen-regulated ornithine decarboxylase mRNAs of mouse kidney. J Biol Chem. 1984 Jun 25;259(12):7941–7946. [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoda L., van Daalen Wetters T., Macrae M., Ascherman D., Coffino P. Prevention of rapid intracellular degradation of ODC by a carboxyl-terminal truncation. Science. 1989 Mar 17;243(4897):1493–1495. doi: 10.1126/science.2928784. [DOI] [PubMed] [Google Scholar]
- Gilmour S. K., Avdalovic N., Madara T., O'Brien T. G. Induction of ornithine decarboxylase by 12-O-tetradecanoylphorbol 13-acetate in hamster fibroblasts. Relationship between levels of enzyme activity, immunoreactive protein, and RNA during the induction process. J Biol Chem. 1985 Dec 25;260(30):16439–16444. [PubMed] [Google Scholar]
- Gilmour S. K., O'Brien T. G. Regulation of ornithine decarboxylase gene expression in normal and transformed hamster embryo fibroblasts following stimulation by 12-O-tetradecanoylphorbol-13-acetate. Carcinogenesis. 1989 Jan;10(1):157–162. doi: 10.1093/carcin/10.1.157. [DOI] [PubMed] [Google Scholar]
- Glass J. R., MacKrell M., Duffy J. J., Gerner E. W. Ornithine decarboxylase production in vitro by using mouse cDNA. Biochem J. 1987 Jul 1;245(1):127–132. doi: 10.1042/bj2450127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm I., Persson L., Stjernborg L., Thorsson L., Heby O. Feedback control of ornithine decarboxylase expression by polyamines. Analysis of ornithine decarboxylase mRNA distribution in polysome profiles and of translation of this mRNA in vitro. Biochem J. 1989 Mar 1;258(2):343–350. doi: 10.1042/bj2580343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovis J. G., Stumpo D. J., Halsey D. L., Blackshear P. J. Effects of mitogens on ornithine decarboxylase activity and messenger RNA levels in normal and protein kinase C-deficient NIH-3T3 fibroblasts. J Biol Chem. 1986 Aug 5;261(22):10380–10386. [PubMed] [Google Scholar]
- Hölttä E., Pohjanpelto P. Control of ornithine decarboxylase in Chinese hamster ovary cells by polyamines. Translational inhibition of synthesis and acceleration of degradation of the enzyme by putrescine, spermidine, and spermine. J Biol Chem. 1986 Jul 15;261(20):9502–9508. [PubMed] [Google Scholar]
- Hölttä E., Sistonen L., Alitalo K. The mechanisms of ornithine decarboxylase deregulation in c-Ha-ras oncogene-transformed NIH 3T3 cells. J Biol Chem. 1988 Mar 25;263(9):4500–4507. [PubMed] [Google Scholar]
- Kahana C., Nathans D. Isolation of cloned cDNA encoding mammalian ornithine decarboxylase. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3645–3649. doi: 10.1073/pnas.81.12.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Translational regulation of mammalian ornithine decarboxylase by polyamines. J Biol Chem. 1985 Dec 15;260(29):15390–15393. [PubMed] [Google Scholar]
- Kameji T., Pegg A. E. Inhibition of translation of mRNAs for ornithine decarboxylase and S-adenosylmethionine decarboxylase by polyamines. J Biol Chem. 1987 Feb 25;262(6):2427–2430. [PubMed] [Google Scholar]
- Katz A., Kahana C. Isolation and characterization of the mouse ornithine decarboxylase gene. J Biol Chem. 1988 Jun 5;263(16):7604–7609. [PubMed] [Google Scholar]
- Katz A., Kahana C. Transcriptional activation of mammalian ornithine decarboxylase during stimulated growth. Mol Cell Biol. 1987 Jul;7(7):2641–2643. doi: 10.1128/mcb.7.7.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol Cell Biol. 1989 Nov;9(11):5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Muller A. J., Witte O. N. The 5' noncoding region of the human leukemia-associated oncogene BCR/ABL is a potent inhibitor of in vitro translation. Mol Cell Biol. 1989 Nov;9(11):5234–5238. doi: 10.1128/mcb.9.11.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Spizz G. Mitogens and protein synthesis inhibitors induce ornithine decarboxylase gene transcription through separate mechanisms in the BC3H1 muscle cell line. Mol Cell Biol. 1986 Aug;6(8):2792–2799. doi: 10.1128/mcb.6.8.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Madhubala R., Kameji T., Bergeron R. J. Control of ornithine decarboxylase activity in alpha-difluoromethylornithine-resistant L1210 cells by polyamines and synthetic analogues. J Biol Chem. 1988 Aug 5;263(22):11008–11014. [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Kaplan G., Racaniello V. R., Sonenberg N. Cap-independent translation of poliovirus mRNA is conferred by sequence elements within the 5' noncoding region. Mol Cell Biol. 1988 Mar;8(3):1103–1112. doi: 10.1128/mcb.8.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Persson L., Holm I., Heby O. Regulation of ornithine decarboxylase mRNA translation by polyamines. Studies using a cell-free system and a cell line with an amplified ornithine decarboxylase gene. J Biol Chem. 1988 Mar 5;263(7):3528–3533. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Thomas A., Spaan W., van Steeg H., Voorma H. O., Benne R. Mode of action of protein synthesis initiation factor eIF-1 from rabbit reticulocytes. FEBS Lett. 1980 Jul 11;116(1):67–71. doi: 10.1016/0014-5793(80)80530-8. [DOI] [PubMed] [Google Scholar]
- Wen L., Huang J. K., Blackshear P. J. Rat ornithine decarboxylase gene. Nucleotide sequence, potential regulatory elements, and comparison to the mouse gene. J Biol Chem. 1989 May 25;264(15):9016–9021. [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Daalen Wetters T., Brabant M., Coffino P. Regulation of mouse ornithine decarboxylase activity by cell growth, serum and tetradecanoyl phorbol acetate is governed primarily by sequences within the coding region of the gene. Nucleic Acids Res. 1989 Dec 11;17(23):9843–9860. doi: 10.1093/nar/17.23.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Daalen Wetters T., Macrae M., Brabant M., Sittler A., Coffino P. Polyamine-mediated regulation of mouse ornithine decarboxylase is posttranslational. Mol Cell Biol. 1989 Dec;9(12):5484–5490. doi: 10.1128/mcb.9.12.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kranen H. J., van de Zande L., van Kreijl C. F., Bisschop A., Wieringa B. Cloning and nucleotide sequence of rat ornithine decarboxylase cDNA. Gene. 1987;60(2-3):145–155. doi: 10.1016/0378-1119(87)90222-8. [DOI] [PubMed] [Google Scholar]
- van Steeg H., van Oostrom C. T., Hodemaekers H. M., van Kreyl C. F. Cloning and functional analysis of the rat ornithine decarboxylase-encoding gene. Gene. 1990 Sep 14;93(2):249–256. doi: 10.1016/0378-1119(90)90232-g. [DOI] [PubMed] [Google Scholar]






