Abstract
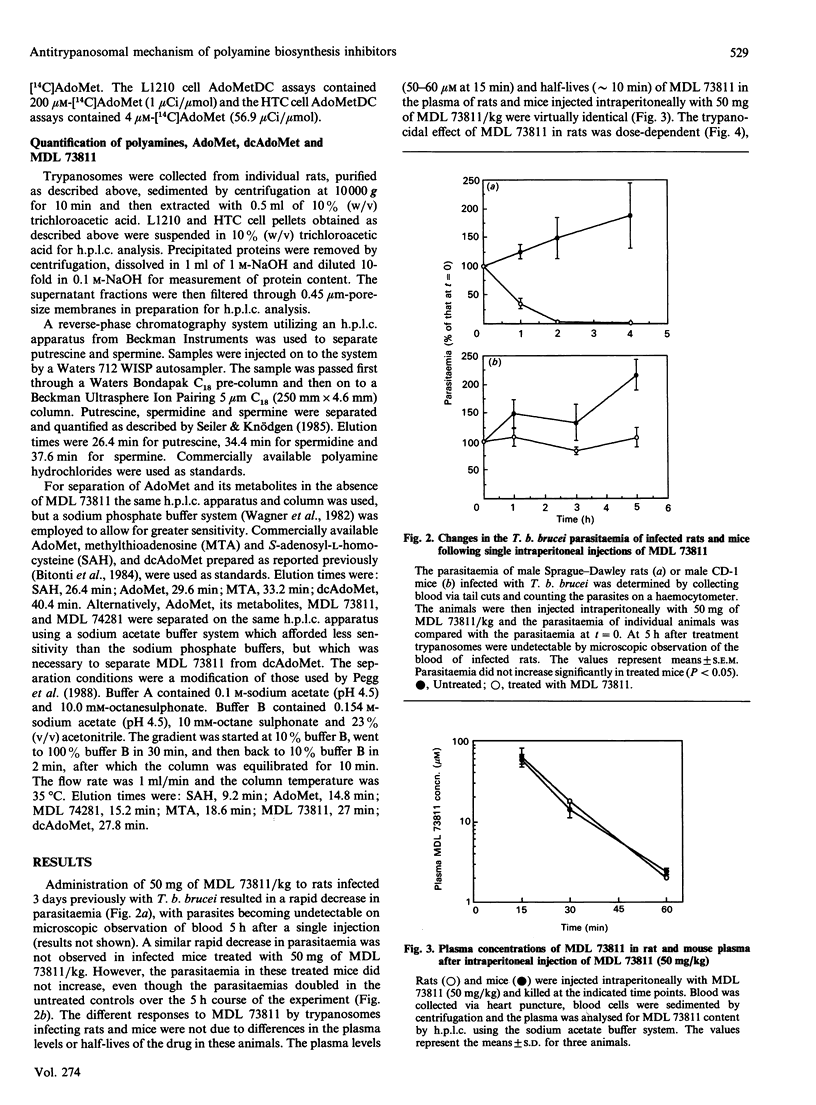
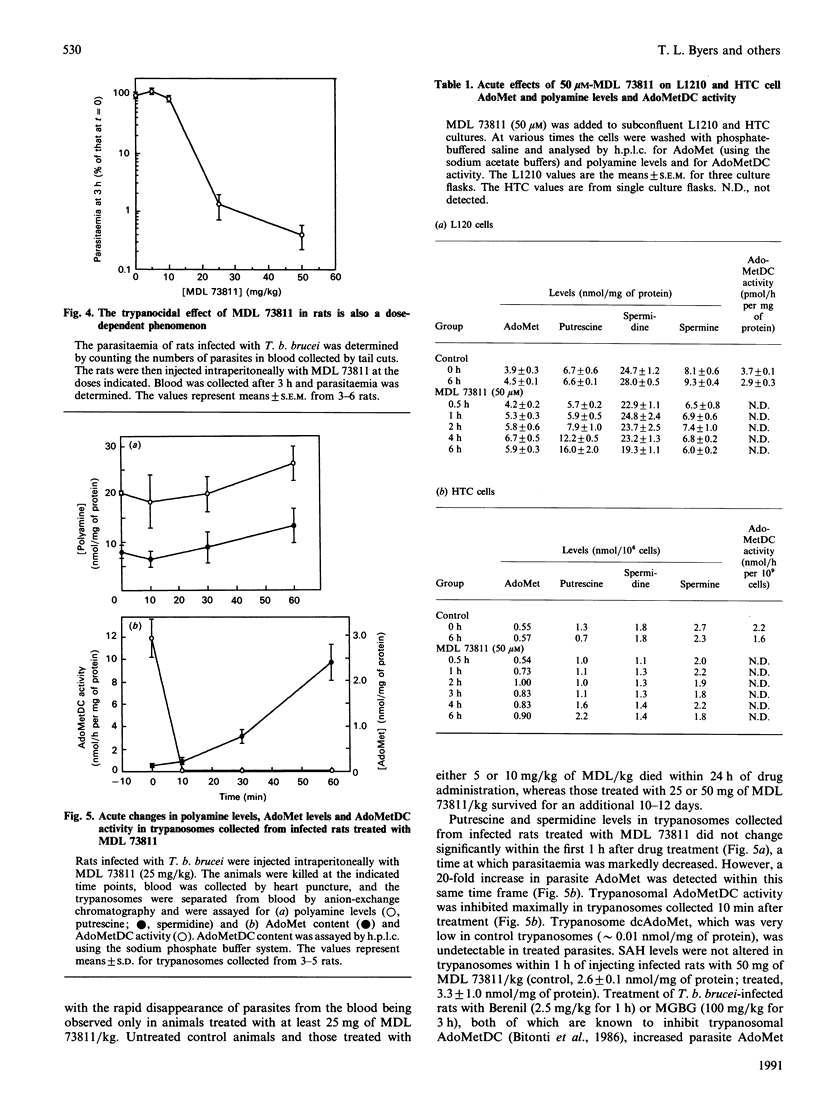
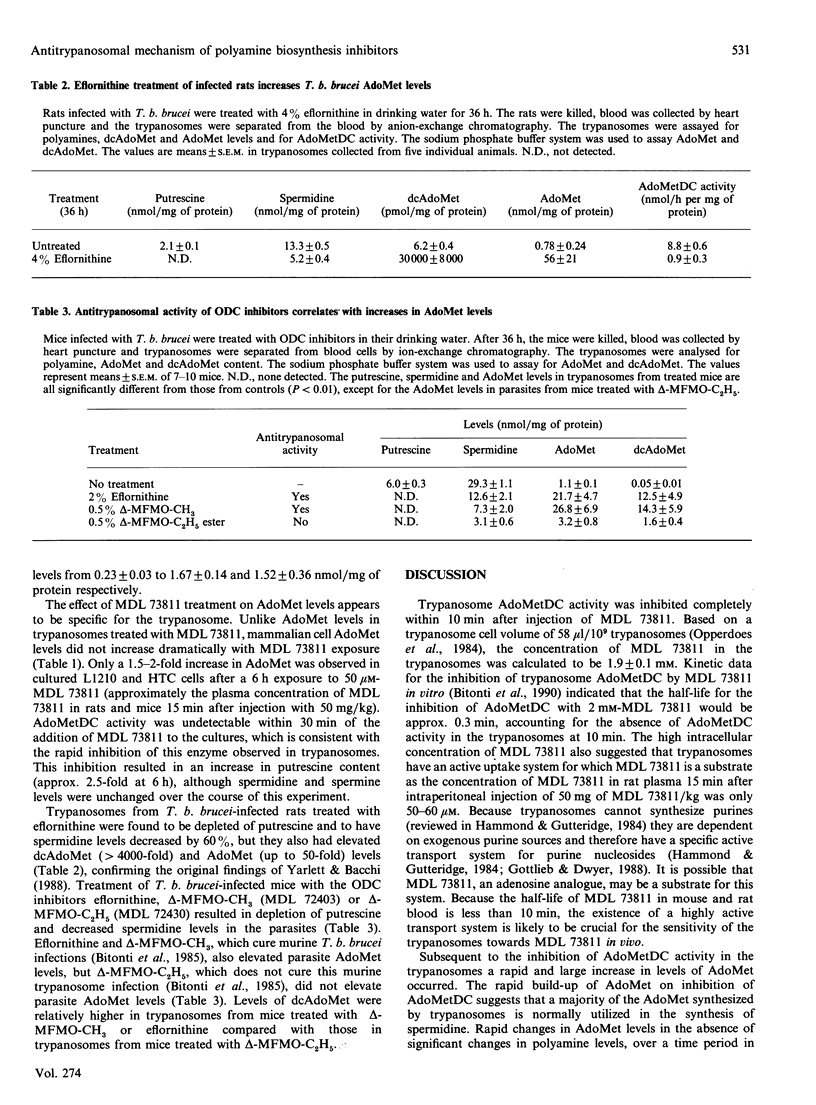
We reported recently that administration of ([(Z)-4-amino-2-butenyl]methylamino)-5'-deoxyadenosine (MDL 73811), an enzyme-activated irreversible inhibitor of S-adenosyl-L-methionine decarboxylase (AdoMetDC; EC 4.1.1.50), a key enzyme in the synthesis of spermidine, cures African trypanosome infections in mice. The precise mechanism of action of MDL 73811 was not clear because a rapid disappearance of trypanosomes from the bloodstream of treated rats occurred before significant depletion of spermidine. Administration of MDL 73811 to Trypanosoma brucei brucei-infected rats resulted in a 70% decrease in parasitaemia within 1 h and a complete disappearance of parasites by 5 h. The reduction in parasitaemia was accompanied by complete inhibition of AdoMetDC activity by 10 min after injection of MDL 73811; inhibition was sustained for at least 4 h. Polyamine levels in trypanosomes were unaffected during the first 1 h in which the marked decrease in parasitaemia was observed, but parasite AdoMet levels increased 20-fold within this time. In contrast, exposure of cultured mammalian cells to MDL 73811 resulted in only a 1.5-2-fold increase in AdoMet levels over a 6 h time course. Experiments with inhibitors of ornithine decarboxylase (ODC) also suggested that the increased AdoMet levels might be an important factor for antitrypanosomal efficacy. Trypanosomes taken from rats treated for 36 h with eflornithine, an inhibitor of ODC, were depleted of putrescine and had markedly decreased spermidine levels. These organisms also had less than 10% of control AdoMetDC activity, and had elevated decarboxy AdoMet (greater than 4000-fold) and AdoMet (up to 50-fold) levels. The methyl ester of alpha-monofluromethyl-3,4-dehydro-ornithine (delta-MFMO-CH3), which cures murine T. b. brucei infections, and the ethyl ester analogue of this compound (delta-MFMO-C2H5), which does not cure this infection, become ODC inhibitors upon hydrolysis and thus were tested for their effects on trypanosomal polyamines, AdoMet and decarboxy AdoMet levels. Although both esters of delta-MFMO depleted trypanosomal polyamines, AdoMet and decarboxy AdoMet levels were elevated in T. b. brucei from infected mice treated with delta-MFMO-CH3 but not in parasites from mice treated with the delta-MFMO-C2H5. These data suggest that inhibition of AdoMetDC, either directly with MDL 73811 or indirectly with inhibitors of ODC, apparently leads to a trypanosome-specific elevation of AdoMet. It is possible that major changes in AdoMet, rather than changes in polyamines, may be responsible for the antitrypanosomal effects of these drugs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchi C. J., Garofalo J., Mockenhaupt D., McCann P. P., Diekema K. A., Pegg A. E., Nathan H. C., Mullaney E. A., Chunosoff L., Sjoerdsma A. In vivo effects of alpha-DL-difluoromethylornithine on the metabolism and morphology of Trypanosoma brucei brucei. Mol Biochem Parasitol. 1983 Mar;7(3):209–225. doi: 10.1016/0166-6851(83)90022-1. [DOI] [PubMed] [Google Scholar]
- Bitonti A. J., Bacchi C. J., McCann P. P., Sjoerdsma A. Catalytic irreversible inhibition of Trypanosoma brucei brucei ornithine decarboxylase by substrate and product analogs and their effects on murine trypanosomiasis. Biochem Pharmacol. 1985 May 15;34(10):1773–1777. doi: 10.1016/0006-2952(85)90648-3. [DOI] [PubMed] [Google Scholar]
- Bitonti A. J., Byers T. L., Bush T. L., Casara P. J., Bacchi C. J., Clarkson A. B., Jr, McCann P. P., Sjoerdsma A. Cure of Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense infections in mice with an irreversible inhibitor of S-adenosylmethionine decarboxylase. Antimicrob Agents Chemother. 1990 Aug;34(8):1485–1490. doi: 10.1128/aac.34.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitonti A. J., Dumont J. A., McCann P. P. Characterization of Trypanosoma brucei brucei S-adenosyl-L-methionine decarboxylase and its inhibition by Berenil, pentamidine and methylglyoxal bis(guanylhydrazone). Biochem J. 1986 Aug 1;237(3):685–689. doi: 10.1042/bj2370685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitonti A. J., Kelly S. E., McCann P. P. Characterization of spermidine synthase from Trypanosoma brucei brucei. Mol Biochem Parasitol. 1984 Sep;13(1):21–28. doi: 10.1016/0166-6851(84)90098-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Cerami A. Identification of a novel, thiol-containing co-factor essential for glutathione reductase enzyme activity in trypanosomatids. Mol Biochem Parasitol. 1985 Feb;14(2):187–198. doi: 10.1016/0166-6851(85)90037-4. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Henderson G. B., Bacchi C. J., Cerami A. In vivo effects of difluoromethylornithine on trypanothione and polyamine levels in bloodstream forms of Trypanosoma brucei. Mol Biochem Parasitol. 1987 Jun;24(2):185–191. doi: 10.1016/0166-6851(87)90105-8. [DOI] [PubMed] [Google Scholar]
- Geller A. M., Kotb M. Y., Jernigan H. M., Jr, Kredich N. M. Purification and properties of rat lens methionine adenosyltransferase. Exp Eye Res. 1986 Dec;43(6):997–1008. doi: 10.1016/0014-4835(86)90077-1. [DOI] [PubMed] [Google Scholar]
- Hammond D. J., Gutteridge W. E. Purine and pyrimidine metabolism in the Trypanosomatidae. Mol Biochem Parasitol. 1984 Nov;13(3):243–261. doi: 10.1016/0166-6851(84)90117-8. [DOI] [PubMed] [Google Scholar]
- Hershko A., Tomkins G. M. Studies on the degradation of tyrosine aminotransferase in hepatoma cells in culture. Influence of the composition of the medium and adenosine triphosphate dependence. J Biol Chem. 1971 Feb 10;246(3):710–714. [PubMed] [Google Scholar]
- Kolb M., Danzin C., Barth J., Claverie N. Synthesis and biochemical properties of chemically stable product analogues of the reaction catalyzed by S-adenosyl-L-methionine decarboxylase. J Med Chem. 1982 May;25(5):550–556. doi: 10.1021/jm00347a014. [DOI] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Danzin C., Kolb M., Gerhart F., Bey P., Sjoerdsma A. Marked and prolonged inhibition of mammalian ornithine decarboxylase in vivo by esters of (E)-2-(fluoromethyl)dehydroornithine. Biochem Pharmacol. 1986 Jan 15;35(2):159–165. doi: 10.1016/0006-2952(86)90509-5. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Danzin C., Wagner J., Siat M., Joder-Ohlenbusch A. M., Claverie N. Accumulation of decarboxylated S-adenosyl-L-methionine in mammalian cells as a consequence of the inhibition of putrescine biosynthesis. Eur J Biochem. 1982 Apr;123(3):499–504. doi: 10.1111/j.1432-1033.1982.tb06559.x. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Joder-Ohlenbusch A. M., Nussli M., Grove J. Indirect evidence for a strict negative control of S-adenosyl-L-methionine decarboxylase by spermidine in rat hepatoma cells. Biochem J. 1981 May 15;196(2):411–422. doi: 10.1042/bj1960411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oden K. L., Carson K., Mecham J. O., Hoffman R. M., Clarke S. S-adenosylmethionine synthetase in cultured normal and oncogenically-transformed human and rat cells. Biochim Biophys Acta. 1983 Oct 18;760(2):270–277. doi: 10.1016/0304-4165(83)90173-3. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R., Baudhuin P., Coppens I., De Roe C., Edwards S. W., Weijers P. J., Misset O. Purification, morphometric analysis, and characterization of the glycosomes (microbodies) of the protozoan hemoflagellate Trypanosoma brucei. J Cell Biol. 1984 Apr;98(4):1178–1184. doi: 10.1083/jcb.98.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Jones D. B., Secrist J. A., 3rd Effect of inhibitors of S-adenosylmethionine decarboxylase on polyamine content and growth of L1210 cells. Biochemistry. 1988 Mar 8;27(5):1408–1415. doi: 10.1021/bi00405a003. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Pösö H. S-adenosylmethionine decarboxylase (rat liver). Methods Enzymol. 1983;94:234–239. doi: 10.1016/s0076-6879(83)94041-7. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. S-adenosylmethionine decarboxylase: a brief review. Cell Biochem Funct. 1984 Jan;2(1):11–15. doi: 10.1002/cbf.290020105. [DOI] [PubMed] [Google Scholar]
- Penketh P. G., Shyam K., Divo A. A., Patton C. L., Sartorelli A. C. Methylating agents as trypanocides. J Med Chem. 1990 Feb;33(2):730–732. doi: 10.1021/jm00164a041. [DOI] [PubMed] [Google Scholar]
- Phillips M. A., Coffino P., Wang C. C. Cloning and sequencing of the ornithine decarboxylase gene from Trypanosoma brucei. Implications for enzyme turnover and selective difluoromethylornithine inhibition. J Biol Chem. 1987 Jun 25;262(18):8721–8727. [PubMed] [Google Scholar]
- Pösö H., Sinervirta R., Jänne J. S-adenosylmethionine decarboxylase from baker's yeast. Biochem J. 1975 Oct;151(1):67–73. doi: 10.1042/bj1510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerdsma A., Schechter P. J. Chemotherapeutic implications of polyamine biosynthesis inhibition. Clin Pharmacol Ther. 1984 Mar;35(3):287–300. doi: 10.1038/clpt.1984.33. [DOI] [PubMed] [Google Scholar]
- Ueland P. M. Pharmacological and biochemical aspects of S-adenosylhomocysteine and S-adenosylhomocysteine hydrolase. Pharmacol Rev. 1982 Sep;34(3):223–253. [PubMed] [Google Scholar]
- Van Nieuwenhove S., Schechter P. J., Declercq J., Boné G., Burke J., Sjoerdsma A. Treatment of gambiense sleeping sickness in the Sudan with oral DFMO (DL-alpha-difluoromethylornithine), an inhibitor of ornithine decarboxylase; first field trial. Trans R Soc Trop Med Hyg. 1985;79(5):692–698. doi: 10.1016/0035-9203(85)90195-6. [DOI] [PubMed] [Google Scholar]
- Wagner J., Danzin C., Mamont P. Reversed-phase ion-pair liquid chromatographic procedure for the simultaneous analysis of S-adenosylmethionine, its metabolites and the natural polyamines. J Chromatogr. 1982 Feb 12;227(2):349–368. doi: 10.1016/s0378-4347(00)80389-8. [DOI] [PubMed] [Google Scholar]
- Yamanoha B., Samejima K. Inhibition of S-adenosylmethionine decarboxylase from rat liver by synthetic decarboxylated S-adenosylmethionine and its analogs. Chem Pharm Bull (Tokyo) 1980 Jul;28(7):2232–2234. doi: 10.1248/cpb.28.2232. [DOI] [PubMed] [Google Scholar]
- Yarlett N., Bacchi C. J. Effect of DL-alpha-difluoromethylornithine on methionine cycle intermediates in Trypanosoma brucei brucei. Mol Biochem Parasitol. 1988 Jan 1;27(1):1–10. doi: 10.1016/0166-6851(88)90019-9. [DOI] [PubMed] [Google Scholar]


