Abstract
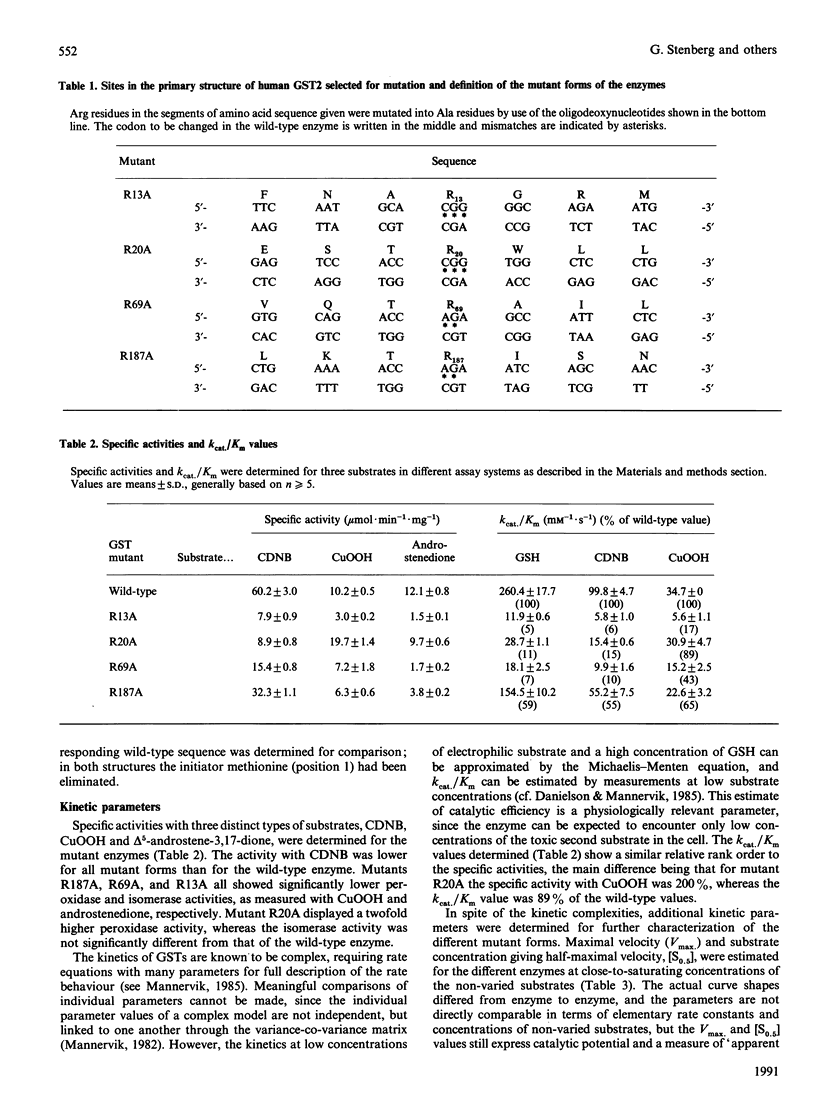
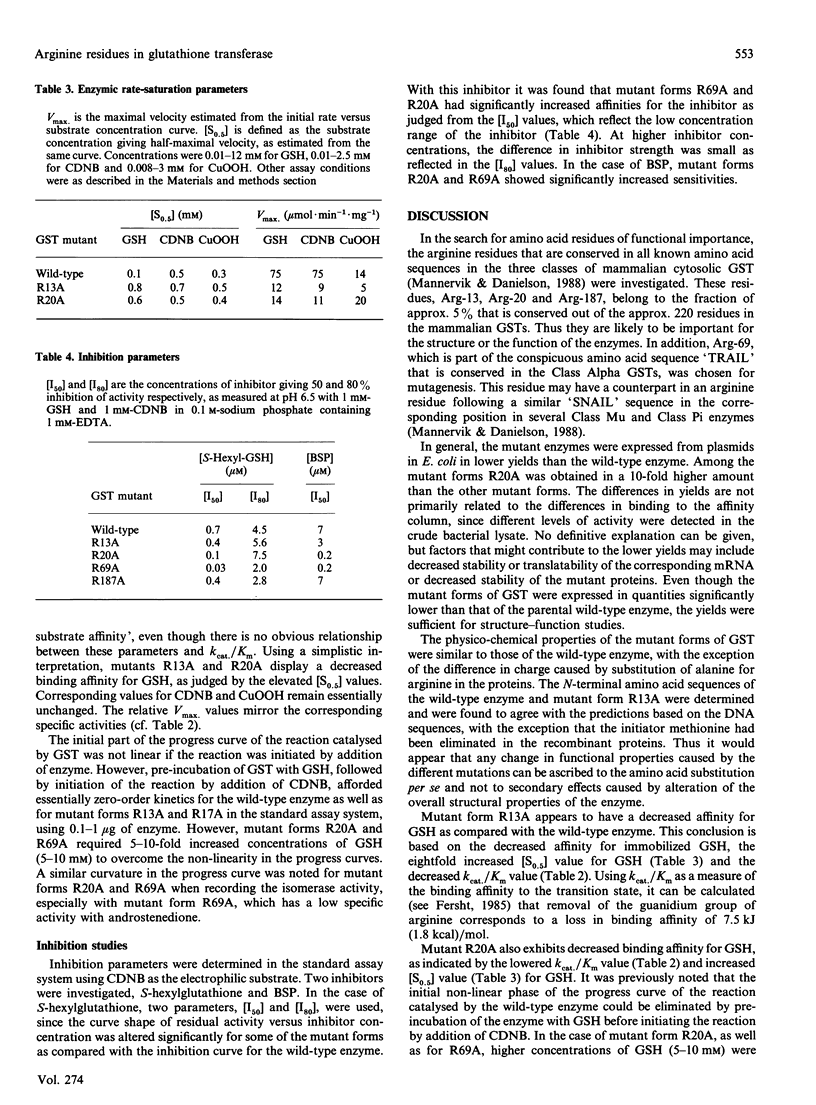
Glutathione transferase (GST) epsilon (also known as GST2 or GST B1B1), the major Class Alpha GST in human liver has been subjected to oligonucleotide-directed site-specific mutagenesis. Four arginine residues, R13, R20, R69 and R187, of which all but R69 are strictly conserved through GST Classes Alpha, Mu and Pi have been replaced by Ala. The mutant enzymes have been expressed in Escherichia coli, purified by affinity chromatography and characterised. Compared with the wild-type enzyme, all mutant GSTs had altered catalytic properties. All mutants had decreased specific activity with 1-chloro-2,4-dinitrobenzene (CDNB). Mutants R13A, R69A and R187A also showed decreased activities with other substrates such as cumene hydroperoxide (CuOOH) and androstenedione. In contrast, mutant R20A had an increased peroxidase activity and an isomerase activity essentially the same as that of the wild-type GST. With the substrates used, kcat./Km values were decreased for all mutant GSTs. Increases in the [S0.5] values were most significant for glutathione (GSH), while values for CDNB and CuOOH were less markedly affected. Thus, various kinetic data indicate that the GSH affinity has been reduced by the mutations and that this loss of affinity is linked to the decreased specific activities. Inhibition studies showed an increased sensitivity towards S-hexyl-GSH; this was particularly marked for mutant R69A. Mutant R20A had a lowered [I50] value but, in contrast, also the highest [I80] value as compared with the wild-type enzyme. Towards bromosulphophthalein, mutants R20A and R69A had a markedly increased sensitivity, about 35-fold in comparison with the wild-type. The inhibition properties of mutant R187A were similar to those of the wild-type enzyme and the properties of mutant R13A were in between. The increased sensitivity to S-hexyl-GSH, in contrast with the decreased affinity for GSH, was suggested to be due to an altered distribution between conformational states of the enzyme induced by the mutations. The arginine residues in positions 13, 20 and 69 all seem to be important for the catalytic properties of GST. Further, the inhibition studies indicate a role of arginine residues in the stabilisation of conformational states of the enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adang A. E., Brussee J., Meyer D. J., Coles B., Ketterer B., van der Gen A., Mulder G. J. Substrate specificity of rat liver glutathione S-transferase isoenzymes for a series of glutathione analogues, modified at the gamma-glutamyl moiety. Biochem J. 1988 Oct 15;255(2):721–724. [PMC free article] [PubMed] [Google Scholar]
- Adang A. E., Meyer D. J., Brussee J., Van der Gen A., Ketterer B., Mulder G. J. Interaction of rat glutathione S-transferases 7-7 and 8-8 with gamma-glutamyl- or glycyl-modified glutathione analogues. Biochem J. 1989 Dec 15;264(3):759–764. doi: 10.1042/bj2640759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson A. M., Talalay P., Keen J. H., Jakoby W. B. Relationship between the soluble glutathione-dependent delta 5-3-ketosteroid isomerase and the glutathione S-transferases of the liver. Proc Natl Acad Sci U S A. 1977 Jan;74(1):158–162. doi: 10.1073/pnas.74.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava M. M., Dasgupta A. Binding of sulfobromophthalein to rat and human ligandins: characterization of a binding-site peptide. Biochim Biophys Acta. 1988 Aug 10;955(3):296–300. doi: 10.1016/0167-4838(88)90207-5. [DOI] [PubMed] [Google Scholar]
- Board P. G. Genetic heterogeneity of the B subunit of coagulation factor XIII: resolution of type 2. Ann Hum Genet. 1984 Jul;48(Pt 3):223–228. doi: 10.1111/j.1469-1809.1984.tb01018.x. [DOI] [PubMed] [Google Scholar]
- Board P. G., Pierce K. Expression of human glutathione S-transferase 2 in Escherichia coli. Immunological comparison with the basic glutathione S-transferases isoenzymes from human liver. Biochem J. 1987 Dec 15;248(3):937–941. doi: 10.1042/bj2480937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan S. W., Bergfors T., Jones T. A., Tibbelin G., Olin B., Board P. G., Mannervik B. Crystallization of GST2, a human class alpha glutathione transferase. J Mol Biol. 1989 Jul 20;208(2):369–370. doi: 10.1016/0022-2836(89)90398-7. [DOI] [PubMed] [Google Scholar]
- Danielson U. H., Mannervik B. Kinetic independence of the subunits of cytosolic glutathione transferase from the rat. Biochem J. 1985 Oct 15;231(2):263–267. doi: 10.1042/bj2310263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli F., Choo K. H., Rees D. J., Boyd Y., Rizza C. R., Brownlee G. G. Gene deletions in patients with haemophilia B and anti-factor IX antibodies. Nature. 1983 May 12;303(5913):181–182. doi: 10.1038/303181a0. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hoesch R. M., Boyer T. D. Localization of a portion of the active site of two rat liver glutathione S-transferases using a photoaffinity label. J Biol Chem. 1989 Oct 25;264(30):17712–17717. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Mannervik B. Regression analysis, experimental error, and statistical criteria in the design and analysis of experiments for discrimination between rival kinetic models. Methods Enzymol. 1982;87:370–390. doi: 10.1016/s0076-6879(82)87023-7. [DOI] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Principato G. B., Danielson U. H., Mannervik B. Relaxed thiol substrate specificity of glutathione transferase effected by a non-substrate glutathione derivative. FEBS Lett. 1988 Apr 11;231(1):155–158. doi: 10.1016/0014-5793(88)80722-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäffer J., Gallay O., Ladenstein R. Glutathione transferase from bovine placenta. Preparation, biochemical characterization, crystallization, and preliminary crystallographic analysis of a neutral class PI enzyme. J Biol Chem. 1988 Nov 25;263(33):17405–17411. [PubMed] [Google Scholar]
- Sesay M. A., Ammon H. L., Armstrong R. N. Crystallization and a preliminary X-ray diffraction study of isozyme 3-3 of glutathione S-transferase from rat liver. J Mol Biol. 1987 Sep 20;197(2):377–378. doi: 10.1016/0022-2836(87)90133-1. [DOI] [PubMed] [Google Scholar]
- Simons P. C., Vander Jagt D. L. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal Biochem. 1977 Oct;82(2):334–341. doi: 10.1016/0003-2697(77)90169-5. [DOI] [PubMed] [Google Scholar]
- Tahir M. K., Mannervik B. Simple inhibition studies for distinction between homodimeric and heterodimeric isoenzymes of glutathione transferase. J Biol Chem. 1986 Jan 25;261(3):1048–1051. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Vince R., Daluge S., Wadd W. B. Studies on the inhibition of glyoxalase I by S-substituted glutathiones. J Med Chem. 1971 May;14(5):402–404. doi: 10.1021/jm00287a006. [DOI] [PubMed] [Google Scholar]



